
Outcome of reduced-dose stereotactic radiosurgery for patients with vestibular schwannoma
Introduction
Vestibular schwannomas (VSs) account for approximately 8% of intracranial tumors in adults and 80–90% of cerebellopontine angle (CPA) tumors. The overall Incidence of VS differs among races. It approximately ranges from 1.3 to 2.66 per 100,000 person-year, with the highest incidence in Taiwan (1-3). VS incidence is increasing because of the widespread use of magnetic resonance imaging (MRI) and the improvement of the national registration system (4-6). The rate of sporadic VS increases with age; the median age at diagnosis is about 50 years old. More than 90% of patients have unilateral tumors (7). Bilateral VS occurs in about 10% of cases, mainly associated with autosomal dominant neurofibromatosis type-2 syndrome (NF2) (8).
Clinically, VS is a type of indolent benign tumor arising from Schwann cells of the vestibulocochlear nerve at the skull base, presenting with a long natural course. Previous studies reported 43–66% of tumors with slow growth of around 0.66–1.9 mm annually; most of the other tumors demonstrated stable disease, and a small portion had regression (1–8%) (9-12). Observational studies found that patients with an accelerated tumor-growth pattern may worsen neurologic symptoms and prompt immediate medical attention (13,14). As a result, regular observation, including serial gadolinium-enhanced MRI with no tumor-directed local treatment, has been suggested for incidental asymptomatic VS (12).
Treatment options of VS include wait-and-scan, microsurgical resection, and radiotherapy (RT), including stereotactic radiosurgery (SRS) or fractionated RT. Professor Leksell treated the first VS patient in 1969 using the gamma knife (GK) system (15). The role of microsurgery became more important after it’s mature. Surgical resection is indicated for young or medically fit patients who demonstrated large VSs with brainstem compression. SRS is widely used for patients with small to medium-sized (<3 cm) without significant mass effect or growing VSs with the main goal of tumor control. Whenever subtotal tumor resection is performed instead of gross tumor resection for cranial nerve function preservation, the risk of tumor regrow is around 30% (16). SRS for post-surgical regrow tumor offers favorable neurological outcomes (12,17). Numerous reports showed that patients treated with a marginal dose of 12–13 Gy achieved tumor control rate (TCR) up to 90–100%. The 5- and 10-year progression-free survival (PFS) were 92–98% (18-23). However, earlier studies before 2000 using irradiation dose of more than 15 Gy showed high complication rates, including trigeminal and facial neuropathies, and hydrocephalus requiring VP shunt management. After SRS, long-term functional hearing preservation was still unsatisfactory (24,25). Although current guidelines recommend a marginal dose of less than 13 Gy, the most common prescription dose range is 12 to 13 Gy. The present study reported our long-term outcomes of low marginal-dose SRS for VS patients, including tumor control and neurological complications. We present the following article in accordance with the STROBE reporting checklist (available at https://tro.amegroups.com/article/view/10.21037/tro-22-15/rc).
Methods
Subjects and populations
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Buddhist Dalin Tzu Chi Hospital (approval number: B11003015) and individual consent for this retrospective analysis was waived. From June 2002 to June 2020, we retrospectively reviewed 50 VS patients who received SRS. Patients were excluded if follow-up time was less than three months or there was incomplete data. A neurosurgeon and two radiation oncologists doubly reviewed all data. Patients’ medical profiles and radiation parameters were retrieved from hard-copy medical charts, the Hospital Information System, and our in-house RT database, the Integrated Radiotherapy Oncology Information Platform (26,27).
Documented baseline data included patient characteristics, tumor-specific profiles, and radiation parameters (e.g., irradiation dosimetry, cochlear dose, and tumor volume). The Koos grading system was used to classify the growth pattern of tumors into four categories: Grade 1, intra-canalicular tumor; Grade 2, tumor extending into the cerebellopontine cistern but not reaching the brainstem and a maximal diameter of 2 cm; Grade 3, contact with the brainstem surface; and Grade 4, a large tumor with brainstem and nerve displacement. Patients were divided into two groups according to the prescribed marginal dose: the standard-dose group (≥12 Gy) and the low-dose group (<12 Gy). Provided that the marginal dose recommended by guidelines is <13 Gy for patients with preserved serviceable hearing, treatment outcomes focus on marginal dose less than 12 Gy is rare because 12–13 Gy is the most common prescription dose range in studies based on the evidence initially proposed by Cleveland Clinic in 2000 (28). Therefore, we selected 12 Gy as our cut-point for further investigation.
Radiosurgery technique
Before the SRS procedure, a neurosurgeon and radiation oncologist co-examined individual patients, including gadolinium-enhanced brain MRI with scanning of 1–3 mm. On the day of treatment, all patients received head-frame fixation under local anesthesia, except two patients who used a bite block and strengthened thermoplastic mask fixation. The planning CT images were fused with MR images to guide delineation for target and organs at risk (OARs), i.e., the brainstem, cochlear, adjacent cranial nerves, and optic pathway. We contoured gross tumor volume (GTV) based on contrast-enhanced T1-weight images. The planning target volume (PTV) had no margin expansion from GTV. Treatment planning was done by a qualified medical physicist using the FastPlan 5.5.1 [2002–2009] or EclipseTM (Varian Medical System, Palo Alto, CA, USA) (after 2010). A marginal dose of 8–15 Gy was prescribed at the 80% isodose line in one isocenter and 70% isodose line in two to three isocenters. The prescribed dose covered at least 95% of the target volume. We prescribed less than 12 Gy in most cases after 2016. Constraints for normal organs were defined as <12 Gy for the brainstem and <9 Gy for the cochlear (in patients with serviceable hearing). We used multiple non-coplanar arcs for beam designing. After an experienced medical physicist generated the SRS plan, a dry run was conducted before treatment. The quality assurance for treatment systems followed the American Association of Physicists in Medicine (AAPM) Task Group-142 report (29).
Radiation was delivered by using 6MV Linear Accelerator (LINAC)-based SRS. We used the Varian 2300IX (Varian Medical System) system for thirteen patients before February 2010 and adopted the Trilogy® with the RapidArc® volumetric modulated arc therapy (VMAT) system (Varian Medical System) for 47 patients after April 2010. Treatment systems were equipped with cone-beam CT scans for image-guided irradiation. To enhance intra-fraction accuracy, we utilized the precise optical guidance platform (OGP) to detect any shift in 0.1 mm. All patients received dexamethasone, metoclopramide, and ranitidine injection one hour before SRS. Furthermore, a second injection was given on the following day.
Outcome evaluations
The follow-up schedule was two weeks, six months, and 12 months after SRS, followed by annual visits subsequently. Physical and neurological examinations were performed on each visit. Gadolinium-enhanced MRIs were performed three to six months after SRS and then in one to two years, depending on the clinical status and physician’s judgment.
The primary endpoint was TCR, which was defined as the absence of symptoms and evidence of tumor growth requiring salvage treatment, including microsurgery and re-irradiation (SRS or fractionated RT). The duration was calculated from SRS to salvage treatment or the last follow-up in patients with no salvage.
Secondary outcome evaluations included radiological tumor control, clinical response, overall survival (OS), and adverse events. For radiological tumor control, tumor size before and after treatment was evaluated using a series of MRIs. We obeyed the consensus criteria proposed by the Seventh International Conference on Acoustic Neuroma to define image tumor control (30). Radiological outcomes were classified into tumor shrinkage (the tumor diameter reduced >2 mm and volume reduced >10%), tumor stability (the diameter reduced <2 mm and volume reduced <10%), and tumor growth (no tumor shrinkage or size-re-increases after an initial shrinkage).
Clinical symptoms and toxicities
Symptoms before and after SRS were evaluated and classified as improvement, stability, or deterioration. Hearing capacity was evaluated by the patient's subjective response because audiometry was not routinely obtained during follow-up. We used the House-Brackmann score to measure the degree of facial palsy: Grade I, normal function; Grade II, slight dysfunction; Grade III, moderate dysfunction; Grade IV, moderately severe dysfunction; Grade V, severe dysfunction; and Grade VI, total palsy. The trigeminal nerve function was assessed using the clinical presentation of facial numbness and pain.
Radiation-induced toxicity was classified according to the Common Terminology Criteria for Adverse Events (CTCAE v5.0). According to the severity of toxicity, Grade 1, 2, 3, 4, and 5 represented mild, moderate, severe of medical significance, life-threatening required urgent intervention, and treatment-related death, respectively. Acute and late toxicities were defined as ≤6 and >6 months after SRS. We recorded a VP shunt procedure after SRS as toxicity.
Statistical analysis
Data were analyzed by using the SAS (version 9.2; SAS Institute, Inc., Cary, NC, USA) and IBM SPSS (version 26, SPSS Inc., Chicago, IL, USA), accordingly. Demographic data were examined between the two groups using the Chi-square test for categorical variables and the Student t-test for continuous variables. TCR and OS were analyzed using the Kaplan-Meier method with the log-rank test. A P value of <0.05 was considered significant statistically.
Results
Patients
Fifty eligible patients were included in the present study. The median age was 58 years (20–82 years). The overall median duration of clinical follow-up was 62 (range, 3–170) months. There were 28 patients in the low-dose group and 22 in the standard-dose group. Patients in the standard-dose group had a longer follow-up duration when compared with those patients in the low-dose group; the median follow-up time was 89 (range, 10–144) and 48 (range, 3–170) months (P=0.005), respectively. The duration of radiological follow-up was also longer in the standard- than low-dose groups, as well: 55 (range, 5–130) versus 25 (range, 2–108) months (P=0.013).
The most common indication for SRS was hearing decline, accounting for 40%, followed by 26% tumor growth on MRI follow-up. Other indications were prevention of hearing deterioration (18%), progression of prior surgery residual disease (10%), and symptom deterioration [e.g., headache (4%), and dizziness (2%)]. The median duration between symptom onset and SRS was longer in the low-dose group (46 months) than in the standard-dose group (6 months). About half of our patients presented with dizziness (n=24, 48%), tinnitus (n=23, 46%), and hearing impairment (n=22, 44%). Four patients (8%) had a loss of fair hearing on the side of the lesion. Facial and trigeminal nerve impairment was presented in one (2%) and four (8%) patients, respectively. Most patients (n=41, 85.4%) received treatment within one year after their MRI-suggested VS.
The extension of VS was evaluated by using pre-SRS MRIs. According to the Koos classification, Grade 1, 2, 3, and 4 tumors were identified in 10 (20%), 15 (30%), 15 (30%), and 10 (20%) patients, respectively. The number of patients who had a Koo Grade 3 tumor was double in the low-dose group (n=10) compared with the standard-dose group (n=5). Patient numbers in other grades was about similar in the two groups. On MRI images, sixteen patients (32%) had tumors with a cystic component. The median maximal tumor diameter and volume were 18.30 (range, 5.0–34.0) mm and 0.9 (range, 0.1–15.1) cm3, respectively, being comparable between the two groups (Table 1).
Table 1
| Items | All (N=50) | <12 Gy (N=28) | ≥12 Gy (N=22) | P |
|---|---|---|---|---|
| Gender | 0.374 | |||
| Male, n [%] | 24 [48] | 15 [30] | 9 [18] | |
| Female, n [%] | 26 [52] | 13 [26] | 13 [26] | |
| Age, median [range], years | 58 [20–82] | 59 [23–82] | 57 [20–77] | 0.593 |
| ≤60, n [%] | 31 [62] | 17 [34%] | 14 [28] | |
| >60, n [%] | 19 [38] | 11 [22%] | 8 [16] | |
| Symptoms to SRS, median [range], months | 7 [0–240] | 46 [3–162] | 6 [0–240] | 0.166 |
| MRI diagnosis to SRS, median [range], months | 2 [0–108] | 2 [1–36] | 1 [0–108] | 0.700 |
| Side of VS, n [%] | 0.569 | |||
| Right | 25 [50] | 15 [30] | 10 [20] | |
| Left | 25 [50] | 13 [26] | 12 [24] | |
| Symptoms for SRS, n [%] | 0.75 | |||
| Tinnitus | 23 [46] | 16 [32] | 7 [14] | |
| Dizziness | 24 [48] | 11 [22] | 13 [26] | |
| Hearing impairment | 22 [44] | 10 [20] | 12 [24] | |
| Imbalance | 8 [16] | 5 [10] | 3 [6] | |
| Trigeminal neuralgia | 4 [8] | 3 [6] | 1 [2] | |
| Facial nerve palsy | 1 [2] | 1 [2] | 0 | |
| Ataxia | 1 [2] | 1 [2] | 0 | |
| Indications of treatment, n [%] | 0.339 | |||
| Growth of tumor | 13 [26] | 6 [12] | 7 [14] | |
| Hearing decline on follow-up | 20 [40] | 11 [22] | 9 [18] | |
| Worsening symptom | 3 [6] | 3 [6] | 0 | |
| Prophylaxis | 9 [18] | 4 [8] | 5 [10] | |
| Progression of prior surgical residual tumor | 5 [10] | 4 [8] | 1 [2] | |
| Koos grade, n [%] | 0.994 | |||
| 1 | 10 [20] | 6 [12] | 4 [8] | |
| 2 | 15 [30] | 7 [14] | 8 [16] | |
| 3 | 15 [30] | 10 [20] | 5 [10] | |
| 4 | 10 [20] | 5 [10] | 5 [10] | |
| Cystic, n [%] | 0.558 | |||
| Yes | 16 [32] | 8 [16] | 8 [16] | |
| No | 34 [68] | 20 [40] | 14 [28] | |
| Tumor diameter, mm | 18.30 [5.0–34.0] | 18.7 (18.59±7.89) | 17 (18.63±7.809) | 0.986 |
| Co-morbidity, n [%] | 26 [52] | 16 [32] | 10 [20] | 0.412 |
| NF2 | 1 [2] | 1 [2] | 0 | |
| DM | 6 [12] | 4 [8] | 2 [4] | |
| Hypertension | 17 [34] | 11 [22] | 6 [12] | |
| Heart disease | 4 [8] | 2 [4] | 2 [4] | |
| Malignancy | 5 [10] | 3 [6] | 2 [4] | |
| Follow-up, median [range], (months) | ||||
| Clinical | 62 [3–170] | 48 [3–170] | 89 [10–144] | 0.005 |
| Radiological | 38 [2–130] | 25 [2–108] | 55 [5–130] | 0.013 |
SRS, stereotactic radiosurgery; MRI, magnetic resonance imaging; VS, vestibular schwannoma; NF2, neurofibromatosis 2; DM, diabetes mellitus; mm, millimeter; Gy, Gray.
Radiation parameters
The median marginal dose was 12 Gy (range, 12–15 Gy) in the standard-dose group and 9 Gy (8–11 Gy) in the low-dose group. The median, mean (Dmean) and maximal dose (Dmax) in the low-dose group (1,235.50 and 1,495.65 cGy) was lower than that of the standard-dose group [1,349.40 cGy (P=0.002) and 1,546.90 cGy (P=0.044)]. The median PTVs for patients treated with the standard and low marginal dose were similar, 0.9 vs. 1.75 cm3 (P=0.549). Dosimetric analysis for the cochlear showed lower Dmean, Dmax, Dmin, and D90 in the low-dose group than that of the standard-dose group (median Dmean =460.70 vs. 575.40 cGy; P=0.046) (Table 2).
Table 2
| Radiation parameters | <12 Gy (N=28) | ≥12 Gy (N=22) | P value |
|---|---|---|---|
| Prescribed dose, median (range), Gy | 9 [8–11] | 12 [12–15] | 0.000 |
| Tumor volume, median (range), cm3 | 1.75 (0.1–12.0) | 0.9 (0.2–15.1) | 0.549 |
| ≤1 cm3 | 12 (24%) | 12 (24%) | |
| >1–2.0 cm3 | 16 (32%) | 10 (20%) | |
| PTV Dmean, median (range), cGy | 1,235.50 (861.50–1,537.70) | 1,349.40 (1,268.40–1,913.40) | 0.002 |
| PTV Dmax, median (range), cGy | 1,495.65 (907.50–1,871.40) | 1,546.90 (1,250.0–2,194.7) | 0.044 |
| Cochlear dose, median (range), cGy | |||
| Dmin | 189.00 (36.90–796.80) | 224.00 (79.50–1,000.0) | 0.277 |
| Dmean | 460.70 (86.60–811.10) | 575.40 (273.7–1,100.00) | 0.046 |
| Dmax | 825.40 (200.60–1,408.50) | 1,000 (420.0–1,327.10) | 0.140 |
| D90 | 261.93 (47.62–581.77) | 327 (201.98–1,080.0) | 0.177 |
PTV, planning target volume; cGy, Centi-Gray; Dmin, minimal radiation dose; Dmean, mean radiation dose; Dmax, maximal radiation dose; D90, dose received by 90% of the treatment volume; Gy, Gray.
TCR
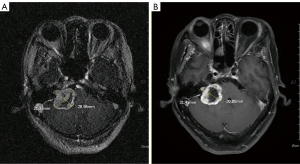
The overall TCR was 98%. The TCR in the standard- and low-dose groups were 100% and 96.4% (P=0.389), respectively. The 5- and 10-year PFS were 98% for all patients (Figure 1). Only one female patient received 10 Gy required salvage microsurgery one year after SRS because of worsening pre-SRS trigeminal neuralgia. Her pre-SRS MRI showed a Koos Grade-4 tumor with a cystic component, 31.3 mm in size, at the right CPA. At the time of salvage treatment, brain MRI showed that the volume of the central cystic component increased (Figure 2). After salvage microsurgery, the patient’s symptoms improved gradually, free from medications for fourteen years.

Radiological response rate
The median duration of radiological follow-up was 38 months, significantly longer for patients in the standard-dose group (55 months) than patients in the low-dose group (25 months). Post-SRS MRIs evaluation showed that most patients demonstrated shrinkage (n=27) and stability of tumor size (n=22). Only one patient who required salvage microsurgery had mild tumor enlargement at re-treatment. In the standard-dose group, tumor shrinkage and stability were 63.7% (n=14) and 36.3% (n=8), no radiological growth. On the other hand, in the low-dose group, tumor shrinkage, stability, and growth were 46.4% (n=13), 50% (n=14), and 3.6% (n=1). Notably, we found that seven patients demonstrated an enlarged tumor size due to edematous change by the first two years after SRS, followed by shrinkage (n=6) and stability (n=1). We noted no significant difference in radiological tumor response rates between the two groups, P=0.465 (Table 3).
Table 3
| Response criteria | The low-dose group (n=28), n (%) | The standard-dose group (n=22), n (%) |
|---|---|---|
| Tumor control | ||
| Shrinkage | 13 (46.4) | 14 (63.7) |
| No swelling | 11 (39.3) | 10 (45.5) |
| Initial swelling | 2 (7.1) | 4 (18.2) |
| Stability | 14 (50.0) | 8 (36.3) |
| No swelling | 14 (50.0) | 7 (31.8) |
| Initial swelling | 0 | 1 (4.5) |
| Progression | ||
| Tumor growth | 1 (3.6) | 0 (0) |
P=0.465 in three-group comparison by using Fisher’s exact test. Tumor control was defined as the absence of symptoms and evidence of tumor growth requiring salvage treatment, including microsurgery and re-irradiation (SRS or fractionated RT). As a result, the tumor control cases included shrinkage and stability diseases [i.e., 13+14 (96.4%) vs. 14+8 (100%), P>0.999 by two-group comparison by using Fisher’s Exact Test]. MRI, magnetic resonance imaging; SRS, stereotactic radiosurgery; RT, radiotherapy.
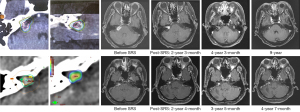
We observed favorable long-term tumor control in two cases who received reduced dose for cystic or post-operative residual lesions. Figure 3 showed a representative MRI of a patient treated with 10 Gy for a cystic lesion over the right CPA. After a nine-year follow-up, the volume of the cystic tumor decreased gradually. The largest dimension and tumor volume were reduced from 27 to 14 mm and 2.8 to 1.2 cm3, respectively (Figure 3, up panels). Another patient received a marginal dose of 8 Gy for post-operative residual VS. MRI at a four-year follow-up revealed tumor shrinkage: the largest diameter decreased from 10.4 to 8.0 mm, and tumor volume reduced from 0.3 to 0.2 cm3 (Figure 3, low panels).
OS
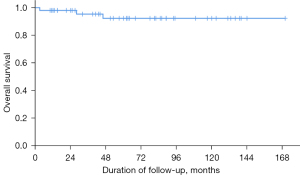
The OS for all patients was 94%. Three patients died at 3, 28, and 46 months after SRS due to pneumonia with respiratory failure, terminal colon cancer, and sudden death due to unknown causes, respectively. No treatment-related death was identified (Figure 4).
Clinical outcome & toxicities
Among the 50 patients, the rate of clinical improvement, stability, and deterioration were 48% (n=24), 40% (n=20), and 2% (n=1), respectively. Five patients (10%) had post-SRS toxicity. The percentage of any grade post-SRS toxicity was lower for low-dose group 7.1% (Grade 1, n=1; Grade 2, n=1), compared with standard group, 13.6% (Grade 1, n=2; Grade 2, n=1), but statistically not significant, P=0.643. In the low-dose group, 14 out of 28 patients gained improvement, 11 gained stability, and one had trigeminal neuralgia deterioration related to tumor growth who was salvaged with microsurgery. Among the 22 patients in the standard-dose group, 10 patients had improvement, 9 patients had stability. One patient in each group developed grade 2 hydrocephalus. The patient of low-dose group received shunt implantation at the 7-month, another patient received procedure at the 15-month. No Grade 3 or greater toxicity was noted. No hearing deterioration was noted among the 46 patients who had a fair hearing before SRS. Overall, trigeminal and facial nerve preservation rates were both 100%. No radionecrosis was found during the follow-up period (Table 4).
Table 4
| Clinical response/treatment toxicity | The low-dose group (N=28) | The standard-dose group (N=22) |
|---|---|---|
| Improvement | 14 (50%) | 10 (45.4%) |
| Stability | 11 (39.3%) | 9 (40.9%) |
| Deterioration | 1 (3.6%)†† | 0 |
| Cranial nerve toxicity† | 0 | 0 |
| Facial nerve | 0 | 0 |
| Trigeminal nerve | 0 | 0 |
| Toxicity (not cranial nerve)† | 2 (7.1%) | 3 (13.6%) |
| Acute (≤6 m) | 1 (G1 dizziness) | 1 (G1 headache) |
| Chronic (>6 m) | 1 (G2 hydrocephalus)* | 1 (G2 hydrocephalus)** 1 (G1 dizziness) |
†, post-SRS toxicities were evaluated by using the CTCAE v5.0, comparing low-dose and standard-dose group, P=0.643 by using Fisher’s exact test. ††, deterioration of pre-existing trigeminal neuropathy after SRS, associated with disease progression, finally treated with salvage microsurgery. Evaluation on toxicities showing hydrocephalus, grade 2, developed in one patient of each group, who required VP shunt implantation at the 7- (*) and 15- month (**) post-SRS. SRS, stereotactic radiosurgery; CTCAE, Common Terminology Criteria for Adverse Events; VP shunt, ventriculoperitoneal shunt.
Discussion
SRS for VS has gained preference over microsurgery in the past half-century because of its favorable cranial nerve preservation and comparable TCR. Frame-based GK is perfectly designed to perform SRS for intracranial lesions, with the strengths of high accuracy down to <0.3 mm, good dose distribution (31,32), and conformity. SRS-capable LINAC was developed in the 1980s, equipped with dynamic high-resolution multileaf collimator, accurate guiding devices, advanced delivery technology, and aided with efficient planning system. SRS can be delivered by using GK- or LINAC-based technique. Several retrospective studies demonstrated comparable outcomes between GK- and LINAC-based SRS techniques (21,24,28,33-40). We offered LINAC-based SRS for all patients. Our TCR was in line with previous large-scaled retrospective series treated with GK, demonstrating 5- and 10-year TCRs of 90–100% and 91–98%, respectively (18,19,21,38,41-43). There is no new onset cranial nerve injury in our cases.
There are no studies to compare the difference in outcome based on radiosurgery equipment (GK vs. LINAC-based SRS vs. proton beam), therefore, no recommendation is proposed (44). A strength of LINAC SRS is able to delivery RT in multiple fractions to reduce the doses of adjacent critical organs, e.g., cochlear dose and brainstem dose. Fractionated RT is encouraged to treat large or irregular shape tumor if surgically unresectable (45). Conventional fractionated regimens using 45–54 in 1.8–2.0 Gy per fraction to PTV margin are recommended (46-48). The 5-year TCR and 5-year hearing function preservation rate were 87–98.5% and 54–94%, respectively (49-58). The introduction of CyberKnife in early 2000 provides another option to treat VS with hypofractionated SRS, using 18 Gy in 3 fractions or 20–25 Gy in 5 fractions. The 5-year TCR and hearing preservation rate were around 94% to 100% and 50% to 87% (50,56,59-62). Two systemic reviews based only on retrospective studies found that facial nerve and trigeminal nerve toxicity were not statistically different between SRS and fractionated RT series (36,40). LINAC-based RT is widely accepted as an effective modality. It is relatively cost-effective and technically accessible for a community hospital to establish a LINAC therapy, compare with GK. In United State, there was a trend showing that patients treated at nonacademic center were nearly two-thirds more likely to receive LINAC-based SRS than GK (63). LINAC-based radiation techniques are evolving to facilitate frame-less SRS and multi-fractionated modality.
The evolution of treatment modalities and diagnostic imaging have changed the treatment landscape of VS over time. There is a trend of increasing use of more conservative strategy for this disease. According to an article of Pollock et al. in 1998, over 30 years after the introduction of SRS, growing use of SRS was observed. The number of patients receiving GK SRS raised from 10 cases in 1987 to 323 cases in 1996. They mentioned that “radiosurgery has a lower morbidity rate, a similar risk of requiring further surgery, and higher patient satisfaction” (64). Well-established evidence suggests that this statement remain true today. Surgical complications were significantly reduced over time because of evolving microsurgical technique, peri-operative care, and intra-operative neurophysiological monitoring. Even though the overall surgical mortality rate declines significantly to 0.2% to 0.38% and associated with lower neurological and vascular complications rate of 8.6% and 1%, respective (65,66), the risk cannot be eliminated. Neurological morbidities, most often hearing and facial nerve function, is directly proportion to tumor size. After microsurgery of tumor size >2.5 cm, the serviceable hearing preservation rate is less than 5%, and the risk of permanent and partial or complete facial nerve paralysis after total resection of large tumor is approximately 50% (67). Two population-based analyses (68,69) and one recently published retrospective study (70) suggest that advanced age (≥65) and comorbidities impact on surgical-related complications, length of stay, and mortality. Therefore, both patients and treating physician favor SRS when local intervention is indicated for small- to medium-size VS. The observational study based on the Surveillance, Epidemiology, and End Results Program (SEER, 2004–2011) and National Cancer Database (NCDB, 2004–2014) database showed a trend of smaller VSs (<2 cm) at diagnosis (68.8%), so, more likely to receive observation. Microsurgery was used less frequent over time, mainly reserved for patients with tumor size more than 3 cm, and the use of RT remained unchanged. Overall, the percentage of patients received observation, radiation, and surgery in 2014 were 34%, 29%, and 37%, respectively (63,71). This analysis would not reflect the situation of other countries due to different medical policy and insurance reimbursement. Nevertheless, the management of VS is evolving and focus on quality of care. The decision of treatment plan should be individualized. Well communication between patient and physician is mandatory.
For VS patients in whom SRS was indicated, our study demonstrated the clinical effectiveness and safety of using a dose reduction strategy, even with a median dose of 9 Gy. Experience from earlier series found that an average marginal dose of 16–18 Gy had poorer hearing and cranial nerve preservation when compared with a relatively low marginal dose of 14–16 Gy. Most of the retrospective studies published before 2000 applied high marginal doses and reported unsatisfactory hearing function, trigeminal, and facial nerve preservation rates of 20–51%, 41.4–85%, and 33.5–86%, respectively. Kondziolka et al. analyzed treatment results from the Pittsburgh Medical Center; a significantly improved hearing preservation rate was found when the marginal dose was reduced from 16–20 to 12–14 Gy (72). Iwai et al. reported that a low-dose SRS of ≤12 Gy at the tumor margin achieved a high TCR of 96% and low post-SRS morbidity, including hearing preservation (66). Mendenhall et al. treated 56 VS patients with LINAC-based SRS with 10–22.5 Gy prescription doses. They observed that the likelihood of complications was related to irradiation dose and treatment volume. Reducing the marginal dose to ≤13 Gy achieved good hearing preservation and less cranial nerve toxicity of <3% (24,73).
Foote et al. reported a comprehensive analysis of risk factors for post-LINAC-based SRS cranial neuropathies at the University of Florida. They evaluated 149 patients who received a mean prescription dose of 14 Gy (10–22.5 Gy) delivered to the 70–80% isodose lines from 1988 to 1998. The overall 2-year actuarial rates of facial and trigeminal neuropathies were 11.8% and 9.5%, respectively. Remarkably, the multiple risk factor model revealed that three parameters predicted cranial neuropathy, including the maximum radiation dose of the brainstem, treatment era (before vs. after 1994), and prior surgical resection. After 1994, a low marginal dose was prescribed to reduce cranial nerve toxicities; the overall radiological TCR was 93% after a 36-month follow-up. When the marginal dose was decreased to 10 Gy, no incidence of cranial nerve palsy was observed; however, it showed a trend toward a low TCR. As a result, they concluded that a prescription dose of 12.5 Gy to the tumor margin resulted in both maximum tumor control and the lowest complication rate (74). A subsequent study reported up to 2005, and 390 patients were retrospectively reviewed. The 5-year actuarial TCR was 90%, with only 1% required salvage surgery for tumor growth. They emphasized that a marginal tumor dose decreased to 12.5, or 10 Gy for large tumors, results in a low rate of cranial neuropathy (e.g., facial weakness or numbness, 0.7%) (36). Flickinger et al. investigated the relationships between irradiation dose and tumor diameter for the risk of developing cranial nerve neuropathies (i.e., trigeminal, facial, and vestibulocochlear nerve). They suggested that cranial nerve neuropathy was associated with transverse tumor diameter and the minimum tumor dose (75). These outcomes further confirmed the importance between dose reduction and cranial nerve preservation. Currently, a single-fraction SRS delivering a marginal dose of less than 13 Gy is recommended by the American Society for Radiation Oncology (ASTRO) Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) Guideline (76), American Association of Neurological Surgeons (AANS) & Congress of Neurological Surgeons (CNS) association (44), European Society for Radiotherapy and Oncology (ESTRO) Advisory Committee on Radiation Oncology Practice (48), and European Association of Neuro-Oncology (EANO) Guidelines (12).
Recent studies including dosimetry analysis suggest favorable functional preservation with lower marginal dose. Watanabe et al. investigated patients who received a reduced marginal dose of ≤12 Gy. The treatment was designed with a unique dose-planning technique and limited to 10 Gy at tumor portions facing the facial and cochlear nerve. No patients experienced additional permanent deterioration of facial function after treatment (23). In the study of Hayhurst et al., among the 200 patients who received a 12 Gy prescription dose, 33.8% of patients developed adverse radiation effects, including trigeminal dysfunction (21%) and facial weakness (3.75%). Further analysis of trigeminal dysfunction found that the maximum dose to the fifth cranial nerve is a significant predictor of its dysfunction, with a threshold of 9 Gy (77).
Hence, we applied the marginal dose reduction strategy to our patients. In the present study, 28 VS patients were treated with a low marginal dose, less than the standard 12 Gy, intending to limit radiation doses to the adjacent cochlea and brainstem. According to the proximity and volume of VS, tumor located adjacent to the cochlea modiolus (<2 mm) or in contact with the brainstem (Koo grade III or IV), we tended to prescribe 8–10 Gy to cover the tumor. Compared with patients treated with a marginal dose of ≥12 Gy, patients with a low marginal dose received a considerably low mean cochlear dose. No complication of the trigeminal or facial nerve was noted. These favorable results encouraged us to prescribe a marginal dose of ≤11 Gy more often after 2016.
Recently, the feasibility of delivering a low marginal dose was widely investigated. Several studies reported their treatment results of 11-Gy SRS. For example, Klijn et al. reported that 420 patients received GK radiosurgery with a median dose of 11 Gy. The 5- and 10-year TCR were 91.3% and 84.8%, respectively (22). Andrew et al. treated 30 consecutive patients with low-dose GK SRS; all patients were prescribed 11 Gy to the 50% isodose line. At five years, the rate of freedom from surgery was 100%, and PFS based on freedom from ongoing growth was 91%. No patients with pre-SRS normal trigeminal and facial function developed subsequent neuropathy. The median duration of follow-up was 42 months. A hearing preservation rate was 50%, and dosimetric analysis suggested that a higher mean (>6 Gy) and maximum dose (>12 Gy) exposed to the cochlea significantly affected hearing function. The author concluded that VS could effectively and safely be treated with low doses (i.e., 11 Gy) by GK-based SRS (78). A recently published study analyzed patients who received LINAC-based SRS with different marginal doses (11–16 Gy) to evaluate whether the dose reduction would affect treatment response and toxicities. A subgroup treated with 11 Gy was particularly reviewed and showed that the 10-year local control rate was over 95%; the permanent facial or trigeminal toxicity rate was below 5%. Univariate and multivariate analysis showed no statistical significance among different doses for local control; however, a low prescribed dose significantly decreased trigeminal and facial nerve toxicities (40).
Current evidence regarding low marginal doses of 8–10 Gy is limited. Several studies mentioned that a small subgroup of patients received marginal doses of 8–11 Gy. However, their outcomes were not analyzed separately (23,28,38,79,80). The median marginal dose of our low-dose group was 9 Gy. This present study is the only study presenting the result of this low-level marginal dose of SRS for VS patients. Our findings agreed that dose reduction demonstrated good cranial nerve preservation and would not compromise the effect of tumor control even with 9 Gy.
The pattern of radiological tumor response for the two groups showed different distribution. Two specific characteristics were found. First, patients in the standard-dose group achieved more tumor shrinkage than those in the low-dose group. The result was not statistically significant because of the small patient number. According to previous long-term studies using a median marginal dose of 12–13 Gy, the percentage of tumor shrinkage, stability, and enlargement based on MRI volumetric evaluation was 44–82%, 7–54%, and 0–12%, respectively (23,81,82). Longer follow-up is required for tumor to shrink. Breshears et al. observed the radiological response of 118 patients after GK SRS, they found that longer follow-up is required for tumor to shrink, that was 45% by 4 year, 77% by 6 year (83). The patient characteristics between the two groups were not significantly different except for the duration of follow-up. Patients in the standard-dose group had a longer follow-up than those in the low-dose group. For irradiated VS patients, tumor response required time mainly due to its indolent disease nature. A shorter follow-up duration may limit us from observing the best response in patients of the low-dose group. However, at the time of evaluation (about two years), the effectiveness of low-dose SRS was still convincible.
The second point to be highlighted in the present study was the population of tumor pseudo-progression (n=7, 14%). According to the definition of transient tumor volume enlargement (i.e., >10% followed by shrinkage), the rate of pseudo-progression was around 10–20% (84). We observed more tumor pseudo-progression in the standard-dose group (n=5) than in the low-dose group (n=2). We found that transit tumor enlargement at a median follow-up duration of 9 months (range, 6–12) on MRI evaluation. Then, tumor stability or shrinkage was subsequently noted with a median time of three years (25–43 months). Hence, it is reasonable to observe if tumor size increased on MRI but with no new onset pathological neuropathy.
Until now, hearing preservation for VS patients is still unsatisfactory. The probability of hearing preservation after SRS was 75–100%, 50–75%, and 25–50% at 2, 5, and 10 years, respectively (12,85). Several factors are proposed to be relevant to hearing preservation, including patients’ age (≥55 years), Gardner-Robertson hearing class before irradiation, Koos tumor grade, internal auditory canal involvement, cochlear dose, and a marginal dose of <13 Gy. Two of these parameters link to RT. Dose prescription and OARs constraints should be carefully evaluated because hearing deterioration may lead to deafness, which significantly impairs a patient’s quality of life and psychosocial activities. Our patients in the low-dose group received a low marginal dose and, therefore, a low mean cochlear dose. Although Gardner-Robertson’s hearing score is not available, our patients with pre-SRS fair hearing did not report subjectively hearing deterioration in their follow-up period. Low hearing toxicity could be expected based on the low radiation exposure to the cochlear. However, long-term follow-up with a prospective standard protocol is required to clarify the clinical effectiveness of hearing function sparing associated with low cochlear dose.
Limitations
The present study has three limitations. First, the retrospective study nature burdens potential biases of patient selection, patient preference, and treatment technique modifications. Second, the discrepancy between duration of clinical and radiological follow-up might affect the outcomes of evaluation. On radiological evaluation, it may take several years for VS tumors to shrink. This may explain the lower tumor shrinkage was found (46.4% vs. 63.7%) for patients in low-dose group, who had shorter follow-up duration (25 vs. 55 months). Previous retrospective studies reported 4-year rate of tumor shrinkage was 45%, comparable with our patients. Besides, longer follow-up period is also important to monitor chronic toxicity. Since the post-SRS complications was found to be correlated with increased of marginal dose, severe chronic toxicity was rare for <13 Gy (19). We need to follow-up patients longer to confirm the long-term safety. Third, the audiological assessment of our cases was incomplete and heterogeneous. Pure-tone audiometry (PTA) and verbal discrimination tests are not obtained routinely and regularly. Because of the lack of standard audiological assessment protocol to evaluate hearing toxicity subjectively, we cannot provide a hearing preservation rate for patients who had a pre-SRS fair hearing.
Furthermore, small case numbers may impair the generalization and validity of our results. However, as mentioned above, previously published studies had only a small portion of patients who received a low-dose regimen of <11 Gy and did not analyze these patients separately. Our results are of value in helping design further prospective clinical trials to confirm the treatment effect and toxicities.
Conclusions
For VS patients, current treatment guidelines recommend a marginal dose of less than 13 Gy with a general 12-Gy prescription. Our results suggested that LINAC-based SRS delivered marginal dose reduction to as low as 9 Gy may be reasonable owing to comparable tumor control without increasing of acute toxicity. There was no new-onset facial nerve or trigeminal nerve complications developed after SRS in our series. Longer duration of follow-up is required for our patients treated with reduced dose because the effects and adverse effects after SRS may take several years to evolve. However, further well-designed prospective studies with a large population are mandated to verify the clinical feasibility of this low-level marginal dose.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tro.amegroups.com/article/view/10.21037/tro-22-15/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tro.amegroups.com/article/view/10.21037/tro-22-15/coif). SKH serves as an unpaid editorial board member of Therapeutic Radiology and Oncology from April 2022 to March 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Buddhist Dalin Tzu Chi Hospital (approval number: B11003015) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Koo M, Lai JT, Yang EY, et al. Incidence of Vestibular Schwannoma in Taiwan from 2001 to 2012: A Population-Based National Health Insurance Study. Ann Otol Rhinol Laryngol 2018;127:694-7. [Crossref] [PubMed]
- Marinelli JP, Nassiri AM, Habermann EB, et al. Underreporting of Vestibular Schwannoma Incidence Within National Brain Tumor and Cancer Registries in the United States. Otol Neurotol 2021;42:e758-63. [Crossref] [PubMed]
- Kleijwegt M, Ho V, Visser O, et al. Real Incidence of Vestibular Schwannoma? Estimations From a National Registry. Otol Neurotol 2016;37:1411-7. [Crossref] [PubMed]
- Cioffi G, Yeboa DN, Kelly M, et al. Epidemiology of vestibular schwannoma in the United States, 2004-2016. Neurooncol Adv 2020;2:vdaa135. [Crossref] [PubMed]
- Marinelli JP, Lohse CM, Grossardt BR, et al. Rising Incidence of Sporadic Vestibular Schwannoma: True Biological Shift Versus Simply Greater Detection. Otol Neurotol 2020;41:813-47. [Crossref] [PubMed]
- Marinelli JP, Lohse CM, Carlson ML. Incidence of Vestibular Schwannoma over the Past Half-Century: A Population-Based Study of Olmsted County, Minnesota. Otolaryngol Head Neck Surg 2018;159:717-23. [Crossref] [PubMed]
- Stangerup SE, Tos M, Caye-Thomasen P, et al. Increasing annual incidence of vestibular schwannoma and age at diagnosis. J Laryngol Otol 2004;118:622-7. [Crossref] [PubMed]
- Smith MJ, Bowers NL, Bulman M, et al. Revisiting neurofibromatosis type 2 diagnostic criteria to exclude LZTR1-related schwannomatosis. Neurology 2017;88:87-92. [Crossref] [PubMed]
- Schnurman Z, Nakamura A, McQuinn MW, et al. Volumetric growth rates of untreated vestibular schwannomas. J Neurosurg 2019; Epub ahead of print. [Crossref] [PubMed]
- Smouha EE, Yoo M, Mohr K, et al. Conservative management of acoustic neuroma: a meta-analysis and proposed treatment algorithm. Laryngoscope 2005;115:450-4. [Crossref] [PubMed]
- Yoshimoto Y. Systematic review of the natural history of vestibular schwannoma. J Neurosurg 2005;103:59-63. [Crossref] [PubMed]
- Goldbrunner R, Weller M, Regis J, et al. EANO guideline on the diagnosis and treatment of vestibular schwannoma. Neuro Oncol 2020;22:31-45. [Crossref] [PubMed]
- Varughese JK, Wentzel-Larsen T, Vassbotn F, et al. Analysis of vestibular schwannoma size in multiple dimensions: a comparative cohort study of different measurement techniques. Clin Otolaryngol 2010;35:97-103. [Crossref] [PubMed]
- Varughese JK, Breivik CN, Wentzel-Larsen T, et al. Growth of untreated vestibular schwannoma: a prospective study. J Neurosurg 2012;116:706-12. [Crossref] [PubMed]
- Leksell L. A note on the treatment of acoustic tumours. Acta Chir Scand 1971;137:763-5. [PubMed]
- Harris MS, Moberly AC, Adunka OF. Partial Resection in Microsurgical Management of Vestibular Schwannomas. JAMA Otolaryngol Head Neck Surg 2017;143:863-4. [Crossref] [PubMed]
- Romiyo P, Ng E, Dejam D, et al. Radiosurgery treatment is associated with improved facial nerve preservation versus repeat resection in recurrent vestibular schwannomas. Acta Neurochir (Wien) 2019;161:1449-56. [Crossref] [PubMed]
- Chopra R, Kondziolka D, Niranjan A, et al. Long-term follow-up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys 2007;68:845-51. [Crossref] [PubMed]
- Murphy ES, Suh JH. Radiotherapy for vestibular schwannomas: a critical review. Int J Radiat Oncol Biol Phys 2011;79:985-97. [Crossref] [PubMed]
- Murphy ES, Barnett GH, Vogelbaum MA, et al. Long-term outcomes of Gamma Knife radiosurgery in patients with vestibular schwannomas. J Neurosurg 2011;114:432-40. [Crossref] [PubMed]
- Benghiat H, Heyes G, Nightingale P, et al. Linear accelerator stereotactic radiosurgery for vestibular schwannomas: a UK series. Clin Oncol (R Coll Radiol) 2014;26:309-15. [Crossref] [PubMed]
- Klijn S, Verheul JB, Beute GN, et al. Gamma Knife radiosurgery for vestibular schwannomas: evaluation of tumor control and its predictors in a large patient cohort in The Netherlands. J Neurosurg 2016;124:1619-26. [Crossref] [PubMed]
- Watanabe S, Yamamoto M, Kawabe T, et al. Stereotactic radiosurgery for vestibular schwannomas: average 10-year follow-up results focusing on long-term hearing preservation. J Neurosurg 2016;125:64-72. [Crossref] [PubMed]
- Mendenhall WM, Friedman WA, Buatti JM, et al. Preliminary results of linear accelerator radiosurgery for acoustic schwannomas. J Neurosurg 1996;85:1013-9. [Crossref] [PubMed]
- Friedman WA. Linear accelerator radiosurgery for vestibular schwannomas. Prog Neurol Surg 2008;21:228-37. [Crossref] [PubMed]
- Yeh PH, Hung SK, Lee MS, et al. Implementing web-based ping-pong-type e-communication to enhance staff satisfaction, multidisciplinary cooperation, and clinical effectiveness: A SQUIRE-compliant quality-improving study. Medicine (Baltimore) 2016;95:e5236. [Crossref] [PubMed]
- Lin YH, Hung SK, Lee MS, et al. Enhancing clinical effectiveness of pre-radiotherapy workflow by using multidisciplinary-cooperating e-control and e-alerts: A SQUIRE-compliant quality-improving study. Medicine (Baltimore) 2017;96:e7185. [Crossref] [PubMed]
- Suh JH, Barnett GH, Sohn JW, et al. Results of linear accelerator-based stereotactic radiosurgery for recurrent and newly diagnosed acoustic neuromas. Int J Cancer 2000;90:145-51. [Crossref] [PubMed]
- Klein EE, Hanley J, Bayouth J, et al. Task Group 142 report: quality assurance of medical accelerators. Med Phys 2009;36:4197-212. [Crossref] [PubMed]
- Wu H, Zhang L, Han D, et al. Summary and consensus in 7th International Conference on acoustic neuroma: An update for the management of sporadic acoustic neuromas. World J Otorhinolaryngol Head Neck Surg 2016;2:234-9. [Crossref] [PubMed]
- Warrington AP, Laing RW, Brada M. Quality assurance in fractionated stereotactic radiotherapy. Radiother Oncol 1994;30:239-46. [Crossref] [PubMed]
- Carminucci A, Nie K, Weiner J, et al. Assessment of motion error for frame-based and noninvasive mask-based fixation using the Leksell Gamma Knife Icon radiosurgery system. J Neurosurg 2018;129:133-9. [Crossref] [PubMed]
- Lee GY, Roos DE, Brophy BP. Radiosurgery for vestibular schwannomas: preliminary results of the Adelaide experience. Stereotact Funct Neurosurg 2001;76:209-12. [Crossref] [PubMed]
- Spiegelmann R, Lidar Z, Gofman J, et al. Linear accelerator radiosurgery for vestibular schwannoma. J Neurosurg 2001;94:7-13. [Crossref] [PubMed]
- Okunaga T, Matsuo T, Hayashi N, et al. Linear accelerator radiosurgery for vestibular schwannoma: measuring tumor volume changes on serial three-dimensional spoiled gradient-echo magnetic resonance images. J Neurosurg 2005;103:53-8. [Crossref] [PubMed]
- Friedman WA, Bradshaw P, Myers A, et al. Linear accelerator radiosurgery for vestibular schwannomas. J Neurosurg 2006;105:657-61. [Crossref] [PubMed]
- Rutten I, Baumert BG, Seidel L, et al. Long-term follow-up reveals low toxicity of radiosurgery for vestibular schwannoma. Radiother Oncol 2007;82:83-9. [Crossref] [PubMed]
- Rueß D, Pöhlmann L, Hellerbach A, et al. Acoustic Neuroma Treated with Stereotactic Radiosurgery: Follow-up of 335 Patients. World Neurosurg 2018;116:e194-202. [Crossref] [PubMed]
- Ellenbogen JR, Waqar M, Kinshuck AJ, et al. Linear accelerator radiosurgery for vestibular schwannomas: Results of medium-term follow-up. Br J Neurosurg 2015;29:678-84. [Crossref] [PubMed]
- Dupic G, Urcissin M, Mom T, et al. Stereotactic Radiosurgery for Vestibular Schwannomas: Reducing Toxicity With 11 Gy as the Marginal Prescribed Dose. Front Oncol 2020;10:598841. [Crossref] [PubMed]
- Flickinger JC, Kondziolka D, Niranjan A, et al. Acoustic neuroma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys 2004;60:225-30. [Crossref] [PubMed]
- Horiba A, Hayashi M, Chernov M, et al. Hearing Preservation after Low-dose Gamma Knife Radiosurgery of Vestibular Schwannomas. Neurol Med Chir (Tokyo) 2016;56:186-92. [Crossref] [PubMed]
- Hasegawa T, Kato T, Naito T, et al. Long-Term Outcomes of Sporadic Vestibular Schwannomas Treated with Recent Stereotactic Radiosurgery Techniques. Int J Radiat Oncol Biol Phys 2020;108:725-33. [Crossref] [PubMed]
- Germano IM, Sheehan J, Parish J, et al. Neurosurgery 2018;82:E49-51. [Crossref] [PubMed]
- Apicella G, Paolini M, Deantonio L, et al. Radiotherapy for vestibular schwannoma: Review of recent literature results. Rep Pract Oncol Radiother 2016;21:399-406. [Crossref] [PubMed]
- Tsao MN, Sahgal A, Xu W, et al. Stereotactic radiosurgery for vestibular schwannoma: International Stereotactic Radiosurgery Society (ISRS) Practice Guideline. J Radiosurg SBRT 2017;5:5-24. [PubMed]
- Pialat PM, Fieux M, Tringali S, et al. Vestibular Schwannoma: Results of Hypofractionated Stereotactic Radiation Therapy. Adv Radiat Oncol 2021;6:100694. [Crossref] [PubMed]
- Combs SE, Baumert BG, Bendszus M, et al. ESTRO ACROP guideline for target volume delineation of skull base tumors. Radiother Oncol 2021;156:80-94. [Crossref] [PubMed]
- Andrews DW, Suarez O, Goldman HW, et al. Stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of acoustic schwannomas: comparative observations of 125 patients treated at one institution. Int J Radiat Oncol Biol Phys 2001;50:1265-78. [Crossref] [PubMed]
- Meijer OW, Vandertop WP, Baayen JC, et al. Single-fraction vs. fractionated linac-based stereotactic radiosurgery for vestibular schwannoma: a single-institution study. Int J Radiat Oncol Biol Phys 2003;56:1390-6. [Crossref] [PubMed]
- Sawamura Y, Shirato H, Sakamoto T, et al. Management of vestibular schwannoma by fractionated stereotactic radiotherapy and associated cerebrospinal fluid malabsorption. J Neurosurg 2003;99:685-92. [Crossref] [PubMed]
- Chan AW, Black P, Ojemann RG, et al. Stereotactic radiotherapy for vestibular schwannomas: favorable outcome with minimal toxicity. Neurosurgery 2005;57:60-70; discussion 60-70. [Crossref] [PubMed]
- Combs SE, Volk S, Schulz-Ertner D, et al. Management of acoustic neuromas with fractionated stereotactic radiotherapy (FSRT): long-term results in 106 patients treated in a single institution. Int J Radiat Oncol Biol Phys 2005;63:75-81. [Crossref] [PubMed]
- Collen C, Ampe B, Gevaert T, et al. Single fraction versus fractionated linac-based stereotactic radiotherapy for vestibular schwannoma: a single-institution experience. Int J Radiat Oncol Biol Phys 2011;81:e503-9. [Crossref] [PubMed]
- Kopp C, Fauser C, Müller A, et al. Stereotactic fractionated radiotherapy and LINAC radiosurgery in the treatment of vestibular schwannoma-report about both stereotactic methods from a single institution. Int J Radiat Oncol Biol Phys 2011;80:1485-91. [Crossref] [PubMed]
- Litre F, Rousseaux P, Jovenin N, et al. Fractionated stereotactic radiotherapy for acoustic neuromas: a prospective monocenter study of about 158 cases. Radiother Oncol 2013;106:169-74. [Crossref] [PubMed]
- Anderson BM, Khuntia D, Bentzen SM, et al. Single institution experience treating 104 vestibular schwannomas with fractionated stereotactic radiation therapy or stereotactic radiosurgery. J Neurooncol 2014;116:187-93. [Crossref] [PubMed]
- Lo A, Ayre G, Ma R, et al. Population-Based Study of Stereotactic Radiosurgery or Fractionated Stereotactic Radiation Therapy for Vestibular Schwannoma: Long-Term Outcomes and Toxicities. Int J Radiat Oncol Biol Phys 2018;100:443-51. [Crossref] [PubMed]
- Puataweepong P, Dhanachai M, Swangsilpa T, et al. Long-term clinical outcomes of stereotactic radiosurgery and hypofractionated stereotactic radiotherapy using the CyberKnife® robotic radiosurgery system for vestibular schwannoma. Asia Pac J Clin Oncol 2022;18:e247-54. [Crossref] [PubMed]
- Morimoto M, Yoshioka Y, Kotsuma T, et al. Hypofractionated stereotactic radiation therapy in three to five fractions for vestibular schwannoma. Jpn J Clin Oncol 2013;43:805-12. [Crossref] [PubMed]
- Tsai JT, Lin JW, Lin CM, et al. Clinical evaluation of CyberKnife in the treatment of vestibular schwannomas. Biomed Res Int 2013;2013:297093. [Crossref] [PubMed]
- Nguyen T, Duong C, Sheppard JP, et al. Hypo-fractionated stereotactic radiotherapy of five fractions with linear accelerator for vestibular schwannomas: A systematic review and meta-analysis. Clin Neurol Neurosurg 2018;166:116-23. [Crossref] [PubMed]
- Bashjawish B, Kılıç S, Baredes S, et al. Changing trends in management of vestibular schwannoma: A National Cancer Database study. Laryngoscope 2019;129:1197-205. [Crossref] [PubMed]
- Pollock BE, Lunsford LD, Norén G. Vestibular schwannoma management in the next century: a radiosurgical perspective. Neurosurgery 1998;43:475-81; discussion 481-3. [Crossref] [PubMed]
- Sughrue ME, Yang I, Aranda D, et al. Beyond audiofacial morbidity after vestibular schwannoma surgery. J Neurosurg 2011;114:367-74. [Crossref] [PubMed]
- Iwai Y, Yamanaka K, Shiotani M, et al. Radiosurgery for acoustic neuromas: results of low-dose treatment. Neurosurgery 2003;53:282-87; discussion 287-8. [Crossref] [PubMed]
- Carlson ML, Link MJ. Vestibular Schwannomas. N Engl J Med 2021;384:1335-48. [Crossref] [PubMed]
- Sylvester MJ, Shastri DN, Patel VM, et al. Outcomes of Vestibular Schwannoma Surgery among the Elderly. Otolaryngol Head Neck Surg 2017;156:166-72. [Crossref] [PubMed]
- Hatch JL, Bauschard MJ, Nguyen SA, et al. National Trends in Vestibular Schwannoma Surgery: Influence of Patient Characteristics on Outcomes. Otolaryngol Head Neck Surg 2018;159:102-9. [Crossref] [PubMed]
- Luryi AL, Babu S, Bojrab DI, et al. Surgical Outcomes After Conservative Resection of Vestibular Schwannoma in the Elderly. Otol Neurotol 2021;42:e1358-61. [Crossref] [PubMed]
- Carlson ML, Habermann EB, Wagie AE, et al. The Changing Landscape of Vestibular Schwannoma Management in the United States--A Shift Toward Conservatism. Otolaryngol Head Neck Surg 2015;153:440-6. [Crossref] [PubMed]
- Kondziolka D, Lunsford LD, McLaughlin MR, et al. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med 1998;339:1426-33. [Crossref] [PubMed]
- Yang I, Sughrue ME, Han SJ, et al. A comprehensive analysis of hearing preservation after radiosurgery for vestibular schwannoma. J Neurosurg 2010;112:851-9. [Crossref] [PubMed]
- Foote KD, Friedman WA, Buatti JM, et al. Analysis of risk factors associated with radiosurgery for vestibular schwannoma. J Neurosurg 2001;95:440-9. [Crossref] [PubMed]
- Flickinger JC, Kondziolka D, Lunsford LD. Dose and diameter relationships for facial, trigeminal, and acoustic neuropathies following acoustic neuroma radiosurgery. Radiother Oncol 1996;41:215-9. [Crossref] [PubMed]
- Kirkpatrick JP, Marks LB, Mayo CS, et al. Estimating normal tissue toxicity in radiosurgery of the CNS: application and limitations of QUANTEC. J Radiosurg SBRT 2011;1:95-107. [PubMed]
- Hayhurst C, Zadeh G. Tumor pseudoprogression following radiosurgery for vestibular schwannoma. Neuro Oncol 2012;14:87-92. [Crossref] [PubMed]
- Schumacher AJ, Lall RR, Lall RR, et al. Low-Dose Gamma Knife Radiosurgery for Vestibular Schwannomas: Tumor Control and Cranial Nerve Function Preservation After 11 Gy. J Neurol Surg B Skull Base 2017;78:2-10. [PubMed]
- Prasad D, Steiner M, Steiner L. Gamma surgery for vestibular schwannoma. J Neurosurg 2000;92:745-59. [Crossref] [PubMed]
- Ogino A, Lunsford LD, Long H, et al. Stereotactic radiosurgery as the first-line treatment for intracanalicular vestibular schwannomas. J Neurosurg 2021;135:1051-7. [Crossref] [PubMed]
- Hsu PW, Chang CN, Lee ST, et al. Outcomes of 75 patients over 12 years treated for acoustic neuromas with linear accelerator-based radiosurgery. J Clin Neurosci 2010;17:556-60. [Crossref] [PubMed]
- Anselmo P, Casale M, Arcidiacono F, et al. Twelve-year results of LINAC-based radiosurgery for vestibular schwannomas. Strahlenther Onkol 2020;196:40-7. [Crossref] [PubMed]
- Breshears JD, Chang J, Molinaro AM, et al. Temporal Dynamics of Pseudoprogression After Gamma Knife Radiosurgery for Vestibular Schwannomas-A Retrospective Volumetric Study. Neurosurgery 2019;84:123-31. [Crossref] [PubMed]
- Bowden G, Cavaleri J, Monaco E III, et al. Cystic Vestibular Schwannomas Respond Best to Radiosurgery. Neurosurgery 2017;81:490-7. [Crossref] [PubMed]
- Morselli C, Boari N, Artico M, et al. The emerging role of hearing loss rehabilitation in patients with vestibular schwannoma treated with Gamma Knife radiosurgery: literature review. Neurosurg Rev 2021;44:223-38. [Crossref] [PubMed]
Cite this article as: Chew CH, Lee MS, Chen JC, Wu TH, Chiou WY, Lin HY, Chen LC, Yeh PH, Hung SK. Outcome of reduced-dose stereotactic radiosurgery for patients with vestibular schwannoma. Ther Radiol Oncol 2022;6:10.


