
Orbital radiotherapy for proptosis in patients with Graves’ ophthalmopathy
Introduction
Graves’ ophthalmopathy (GO), also known as Graves’ orbitopathy, is an autoimmune disease of the retro-ocular tissues. It is typically seen in patients with Graves’ disease (GD) and sometimes in patients with chronic autoimmune thyroiditis in a euthyroid or hypothyroid state. Its clinical features include eyelid retraction, erythema, periorbital and conjunctival edema, strabismus, and proptosis (1).
A pathomechanism underlying GO involves orbital fibroblast activation induced by T cells and other mononuclear immune cells infiltrating the orbit (2). Other mechanisms and details are provided in a review (3). Patients with GO are assessed in terms of disease activity and severity score, including the clinical activity score (CAS) (4), NOSPECS classification (5), European Group on Graves’ Orbitopathy (EUGOGO) classification (6), and the vision, inflammation, strabismus, and appearance classification system (7). GO is treated by correcting hyperthyroidism, relieving ocular symptoms, reducing periorbital inflammation and swelling, and restoring the visual axis and vision, along with encouraging smoking cessation. Mild disease without progression may be treated with local therapy, such as artificial tears and topical steroid eye drops. For moderate to severe disease, if in an active stage, immunomodulatory therapy or orbital radiotherapy (ORT) is usually indicated. Oral or intravenous corticosteroids have been used as first-line immunosuppressors for decades in patients with active GO; however, a side effect, known as corticosteroid withdrawal, in GO patients has been noted during treatment courses or after completion. In the inactive stage of moderate to severe GO, because the enlarged extraocular muscle (EOM) and excessive orbital soft tissue do not regress, rehabilitative surgery might be indicated, usually in a staged fashion (8).
ORT has been used for GO for approximately 80 years (9), and its efficacy has been long debated. In 2012, a Cochrane meta-analysis including five randomized controlled trials (244 participants) evaluated the efficacy of ORT and concluded that the regression rate—defined as improvement in diplopia, visual acuity, soft tissue, or proptosis—of patients who received ORT was 1.92 times higher than that of patients who did not. A combination of ORT and steroids may yield better outcomes than steroids alone (10). Reported effective doses of ORT range widely from 2.4 to 30 Gy, such as 20 Gy in 10 fractions over 2 weeks, 10 Gy in 10 fractions over 2 weeks, 20 Gy in 20 fractions over 20 weeks, 16 Gy in 8 fractions, and 2.4 Gy in 8 fractions (11-23).
In Taiwan, ORT is fully covered by National Health Insurance, whereas most target-specific immunomodulators are not. Thus, we considered ORT to be an alternative incurring a lower economic burden on patients with GO. Plus, at our institute, many patients with moderate to severe GO prefer nonsurgical treatment due to concerns about surgery and surgical complications; in particular, those with active-phase GO can experience rapid adverse cosmetic changes and desire immediate management.
Accordingly, ORT is arranged in a timely manner after patients visit our clinic. Among the wide selection of effective doses, we chose 10 Gy in 10 fractions over 2–3 weeks over the commonly used 20 Gy because it causes fewer side effects. We prefer the 2–3-week treatment period because it has superior compliance to that of a 20-week course (22). In our institute, the main concern for patients with moderate to severe GO seeking treatment is proptosis. Severe cases of proptosis involve disfigurement, corneal ulceration, and optic neuropathy, causing cosmetic and visual morbidity. We therefore evaluated the efficacy of ORT in treating proptosis in Taiwanese patients with GO. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/tro-21-10).
Methods
Patient population
This retrospective study included patients with GO and proptosis who received bilateral ORT (10 fractions of 1 Gy over 2–3 weeks). All patient data, including sex, smoking habit, signs of active GO, types of GO, duration of GO symptoms, thyroid function before ORT, EOM enlargement, first dates of ORT, and side effects were retrieved from the medical records of Shin Kong Wu Ho-Su Memorial Hospital in Taipei City, Taiwan, from April 2007 to March 2020. The indications for ORT referral were (I) active and moderate to severe GO with proptosis, irrespective of thyroid function and (II) inactive and moderative to severe GO with rapid progression and proptosis in the euthyroid state for more than 1 year, with patient refusal to undergo surgery. The activity and severity of GO were retrieved from the medical records. The degree of proptosis (mm) was measured by the same ophthalmologist with a Hertel exophthalmometer (Inami & Co., Tokyo, Japan) before and after ORT during regular follow-up visits. We excluded patients receiving steroids 12 months prior to RT, concurrent steroids, or rituximab, and those lost to follow-up. This study was approved by the Institutional Review Board of Shin Kong Wu Ho-Su Memorial Hospital (20190407R), along with a waiver of consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Orbital radiotherapy and treatment techniques
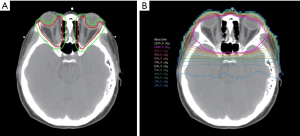
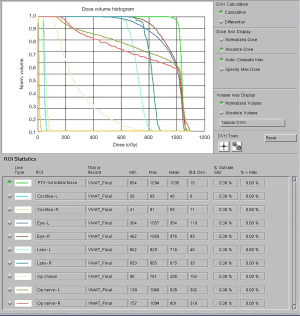
Each patient’s head was immobilized with a thermoplastic mask on a fixed-height rigid headrest. A computed tomography (CT) scan of the orbital fossa was performed for treatment planning. The target for ORT, or the clinical treatment volume (CTV), included the bilateral orbital fossa, comprising EOMs and retrobulbar soft tissues. Bilateral ORT was given irrespective of the laterality of proptosis. A 5-mm margin allowance for daily radiotherapy set-up error was added to the CTV for generating the planning treatment volume (PTV). PTV was administered in 1-Gy fractions delivered 5 days a week for a total dose of 10 Gy over 2–3 calendar weeks. The CTV, PTV, and dose distribution were as depicted (Figure 1). A dose-volume histogram was shown in Figure 2. Patient position was aligned with treatment plan utilizing 0° and 90° port films acquired 1 day before ORT initiation. ORT was planned and delivered using volumetric modulated arc therapy (VMAT) and 6-MV photons using a Pinnacle treatment planning system. The dose administration was optimized to avoid the brain and the lens of the eye. Daily isocenter alignment was achieved by using marks attached to a thermoplastic cast.
Variable definition and statistical analysis
Regression/improvement, nonprogression, and progression of proptosis were defined as ≥1 mm of regression, nonprogressive change of <1 mm, and ≥1 mm of progression, respectively. Post-ORT degree of proptosis was defined as the follow-up exophthalmometry measurements minus the first exophthalmometry measurements before ORT. Post-ORT duration was defined as the duration between follow-up dates and the first dates before ORT. Hyperthyroid function status was defined as elevated free T4 level within 1 month before ORT. Activity of GO was based on Clinical Activity Score (CAS), active defined as CAS ≥3/7. Severity of GO was based on EUGOGO classification, where moderate to severe GO was defined as any of the followings: lid retraction (>2 mm), moderate or severe soft tissue involvement, exophthalmos (≥3 mm), and inconstant or constant diplopia (6). Types of GO, including predominantly fat compartment enlargement (type I) and predominantly EOM enlargement (type II) (24), were based on formal CT imaging reports by radiologists.
Measurements of proptosis by a Hertel exophthalmometer were performed a median of four times (range: 2–7), totaling 101 patient points during the follow-up period. Continuous data are expressed as mean and standard deviation (SD) or 95% confidence interval (CI), and categorical data are expressed as number and percentage. Examination of the normal quantile-quantile plot showed a non-normal distribution in measurements of post-ORT duration; thus, the mean, median, interquartile range (IQR), minimum, and maximum are shown. Student T-test was used to determine the difference of post-ORT degree of proptosis between the left and right eyes. For continuous post-ORT degree of proptosis, linear mixed regression with exchange matrix was used to examine the correlations from repeated measures. The coefficients (effect), standard errors of the coefficients, and P values are presented. Data missing was excluded from analysis. Multivariable analysis of sex, smoking habit, duration of GO symptoms, thyroid function status before ORT, EOM enlargement, and post-ORT duration was conducted in final models. Subgroup analysis was conducted in all variables with linear mixed regression. Statistical analysis was performed using SPSS 26.0.0.0. A P value of <0.05 was set as statistically significant.
Results
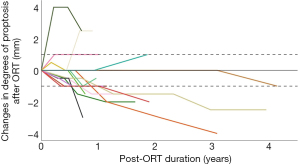
We included 23 patients (12 men and 11 women) who received bilateral ORT (mean age: 51.1±26 years; range: 21–72). Of the 23 patients, 5 (22%) were smokers, 17 (74%) were in active stage of GO, 23 (100%) were moderate to severe GO, 5 (23%) were predominantly fat compartment enlargement, 17 (77%) were predominantly EOM enlargement (one patient had no CT image available), 3 (13%) were in hyperthyroid status within 1 month before the start of ORT, and 7 (30%) had a proptosis-to-ORT duration of ≤6 months (Table 1). The initial mean measurement of proptosis of the right eye was 19.4±3.7 mm. The following statistical analysis is presented with the right eye because no difference in proptosis was observed between the left and right eyes before treatment (t test, P=0.114). A spider plot depicting the degree of proptosis and the duration of follow-up up to 4 years revealed that 3 patients had progression of proptosis within 1 year after ORT, and 17 (85%) had nonprogressive status 1 year after ORT, and only 4 patients had progression throughout follow-up. A trend of favorable treatment response to ORT was observed (Figure 3).
Table 1
| N/total or mean/median | Percentage or SD/IQR | Min | Max | |
|---|---|---|---|---|
| Gender: male | 12/23 | 52% | ||
| Smoking | 5/23 | 22% | ||
| Active GO | 17/23 | 74% | ||
| Severity: moderate to severe | 23/23 | 100% | ||
| EOM enlargement on CT† (missing n =1) | 17/22 | 77% | ||
| Hyperthyroid status | 3/23 | 13% | ||
| Proptosis-to-ORT duration ≤6 months | 7/23 | 30% | ||
| Last measurement after ORT (months) | 18.9/14.0 | 1.23/11.5 | 12.7 | 50.4 |
| Age (years) | 51.1 | 25.9 | 21 | 72 |
| Initial degree of proptosis (right eyes, mm) | 19.4 | 3.7 | 15 | 24 |
| Initial degree of proptosis (left eyes, mm) | 19.2 | 5.0 | 14 | 24 |
†, same population as type II GO.
The relationships between sex, smoking habits, duration of GO symptoms, thyroid function before ORT, EOM enlargement, and post-ORT duration and degree of proptosis after treatment were analyzed. ORT was effective in reducing proptosis in this cohort overall. Multivariable analysis showed that among those with ≤6-month history of proptosis (P<0.001), smoking (P<0.001), a euthyroid state before ORT (P<0.001), no EOM enlargement (P<0.001), and female sex (P<0.01) yielded regression coefficients of −1.84, −2.21, −1.89, −2.06, and −2.10, respectively, suggesting that a patient with these characteristics would expect, on average, a regression/improvement in proptosis by the absolute value of the above coefficients (Table 2). To sum up, GO symptoms for ≤6 months, history of having a smoking habit, a euthyroid state, female sex, and no EOM enlargement were factors significantly favorable to ORT reducing GO-related proptosis.
Table 2
| Coefficient | 95% CI | P value | |
|---|---|---|---|
| Proptosis-to-ORT duration ≤6 months | −1.84 | −2.839, −0.849 | 0.0003*** |
| Smoking | −2.21 | −3.505, −0.912 | 0.0008*** |
| Hyperthyroid status | 1.89 | 1.239, 2.535 | 0.0000*** |
| EOM enlargement on CT | 2.06 | 1.212, 2.914 | 0.0000*** |
| Male sex | 2.10 | 0.549, 3.644 | 0.0079** |
| EOM hypertrophy x male sex interaction | −2.27 | −3.898, −0.644 | 0.0062** |
| Post-ORT duration in years | −0.53 | −0.961, −0.091 | 0.0178* |
Negative values represent regression/improvement of proptosis by millimeter, *, P<0.05; **, P<0.01; ***, P<0.001, the model is adjusted for proptosis-to-ORT duration, smoking habit, thyroid function status before ORT, EOM enlargement, sex, and post-ORT duration. Analyses of proptosis-to-ORT durations of ≤6, 9, 12 months were conducted and only 6 months reached statistical significance; analysis of 3 months was not conducted because of insufficient data (only three cases).
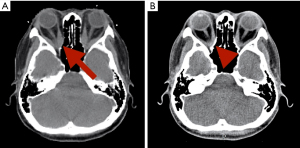
Subgroup analysis was conducted for smoking, hyperthyroid status, EOM enlargement on CT, male sex, and post-ORT duration in years, respectively. It was significant only for subgroups based on proptosis-to-ORT duration. The analysis between the patients with a proptosis-to-ORT duration of ≤6 and >6 months to ORT treatment response revealed results consistent with those of the overall analysis for all but one variable (Table 3). Among those with ≤6 months of proptosis, CT-confirmed EOM enlargement was associated with, on average, greater proptosis regression by 1.14 mm, 95% CI: 0.482, 1.789 (P<0.001); conversely, among those with >6 months of proptosis, CT-confirmed EOM enlargement was associated with an even greater proptosis progression, by 2.27 mm on average, 95% CI: 2.020, 2.186 (P<0.001). The overall regression and nonprogression rates were 5% and 91% at 3 months, 30% and 90% at 6 months, 45% and 85% at 1 year, and 56% and 89% at 2 years after ORT (Table 4). Comparison of CT images before and after ORT revealed prominent regression of proptosis in a patient with EOM enlargement 2.5 years after ORT (Figure 4). No patient experienced any grade of corneal ulcer, conjunctivitis, skin reaction, vision-related complications, or any secondary malignancies.
Table 3
| Parameter | Coefficient | 95% CI | P value |
|---|---|---|---|
| Onset of proptosis to ORT duration >6 months | |||
| Smoking | −1.89 | −3.161, −0.624 | 0.0035** |
| Hyperthyroid status | 2.10 | 2.020, 2.186 | 0.0000*** |
| EOM enlargement on CT | 2.27 | 1.401, 3.137 | 0.0000*** |
| Male sex | 1.34 | 0.057, 2.619 | 0.0406* |
| EOM enlargement x male sex interaction | −1.88 | −3.496, −0.267 | 0.0216* |
| Post-ORT duration in years | −0.57 | −1.054, −0.084 | 0.0223* |
| Onset of proptosis-to-ORT duration ≤6 months | |||
| Smoking | 0‡ | NA | NA |
| Hyperthyroid status | 2.24 | 1.806, 2.666 | 0.0000*** |
| EOM enlargement on CT | −1.14 | −1.789, −0.482 | 0.0007*** |
| Male sex | 0.60 | 0.044, 1.148 | 0.0343* |
| EOM enlargement x male sex interaction | 0‡ | NA | NA |
| Post-ORT duration in years | −0.33 | −0.645, −0.004 | 0.0469* |
Negative values represent regression/improvement of proptosis by millimeter, *, P<0.05; **, P<0.01; ***, P<0.001. ‡, redundant; NA, not available, the model is adjusted for smoking habit, thyroid function status before ORT, EOM enlargement, sex, and post-ORT duration.
Table 4
| Follow-up | 3 months | 6 months | 1 year | 2 years |
|---|---|---|---|---|
| Case number | 22 (96%) | 20 (87%) | 20 (87%) | 9 (39%) |
| Days | 109 (37)§ | 202 (53)§ | 369 (64)§ | 702 (86)§ |
| Regression | 1 (5%) | 6 (30%) | 9 (45%) | 5 (56%) |
| Progression | 2 (9%) | 2 (10%) | 3 (15%) | 1 (11%) |
| Non-progression | 20 (91%) | 18 (90%) | 17 (85%) | 8 (89%) |
Presented as cross-sectional data, §, data presented as mean (SD).
Discussion
This is the first report in Taiwan using ORT with 1 Gy per fraction for 10 fractions to treat moderate to severe GO patients with proptosis. Hsu et al. reported an 83.3% improvement in proptosis after ORT with 20 Gy in 10 fractions in a retrospective study of 19 patients in Taiwan (25). However, they did not specifically define improvement. Grassi et al. conducted a prospective randomized study with 35 patients receiving ORT and reported no significant change in proptosis for 12 months after ORT (26). However, they defined improvement as ≥2-mm regression, which may underestimate the percentage of GO regression, compared with the definition of ≥1-mm regression that was used in our study. Another factor impeding proptosis improvement in their study may be the heterogeneity of proptosis components (soft tissue swelling, EOM enlargement, or both), which are common in patients with GO (26). Li et al. evaluated 78 patients with proptosis who received bilateral ORT at 2 Gy per fraction for 10 fractions over 2–3 weeks. Of them, only 10% and 41% of patients had complete and partial improvement of proptosis, respectively, 6 months after ORT. Their study did not describe the grading system for proptosis in detail. However, they indicated that 73% of patients with GO had benefited from ORT within 6 months, 95% of whom had long-term maintenance effects (median follow-up period: 62 months). Furthermore, 18% of their patients received concurrent steroids, but no synergistic effect of steroid and ORT was observed in the results (27). This is somewhat comparable to our 30% regression and 90% nonprogression rates at 6 months after ORT. Sisti et al. (28) reported that a combination of ORT (20 Gy in 10 fractions) and intravenous corticosteroids for moderate to severe GO led to an overall improvement in 67.7% of patients and 29.1% of patients were stable. They defined improvement as any of ≥2 mm regression of proptosis, CAS reduction of ≥1/7 points, regression in lid retraction, and improvements in diplopia. All their patients underwent ORT within 6 months of GO symptoms, and 63% of them were euthyroid during ORT (28). The combination of steroid and ORT may lead to more favorable outcomes than ORT only, which was the case in our study. Limited literature was available for ORT at 1 Gy per fraction for 10 fractions. Kahaly et al. reported similar response rates, defined as improvement in any three of lid fissure width, proptosis, intraocular pressure, diplopia, and EOM enlargement, for 1- and 2-Gy fractions for 10 fractions (22).
We believe it is reasonable to choose 1 mm instead of 2 mm as the response threshold for several reasons. First, the mean and upper limits of exophthalmetric values for normal adults were fundamentally lower for Chinese in Taiwan than those of Caucasian and African American, likely due to rounder and shallow orbit in Asian people (29,30). Moreover, Hertel exophthalmometry may underestimate exophthalmos in GO patients with orbital edema (31). Plus, improving discomfort and subjective appearance were noted in patients with ≥1 mm regression of proptosis. Also, a 0.6–0.9 mm decrease in proptosis was reported 6 months after ORT (23).
Our results from the subgroup analysis of proptosis-to-ORT duration showed a better reduction of proptosis in patients treated within 6 months of GO presentation. Therefore, our data imply that the best treatment effect with ORT may be within 6 months of GO symptoms with CT-confirmed EOM enlargement. This may be because within 6 months of GO symptoms, the retro-orbital tissues, including EOM, may still be in an acute or subacute inflammatory stage. In the early stages of GO, type 1 helper T cells produce interferon-ɣ and tumor necrosis factor (TNF)-α, and macrophages produce interleukin (IL)-1 (32), stimulating orbital fibroblasts to produce prostaglandin (PG) E2 and hydrophilic hyaluronan (HA), which may accumulate between EOM fibers, causing EOM enlargement and periorbital edema (24,33). After 6 months, however, preadipocytes, which constitute a portion of orbital fibroblasts, may differentiate into mature adipocytes because of PGs produced by activated T cells (34), thereby enhancing de novo adipogenesis and increasing resistance to ORT. Moreover, HA accumulation in the EOM makes it more fibrotic and refractory to ORT. Thus, ORT is recommended to be administered within 6 months of proptosis.
Progression of proptosis after ORT was noted in four patients with initially active GO. The first case underwent orbital decompression surgery to further reduce proptosis, but proptosis and diplopia recurred soon afterwards. The second case underwent radioactive iodine (RAI) ablation at outside hospital because of uncontrolled long-standing hyperthyroidism. The third case had normal thyroid function with persistent high-titers thyrotropin-related antibodies during follow-up. The last case experienced a relapse of hyperthyroidism. Following oculoplastic surgery, GO reactivation might be triggered due to retroorbital inflammatory response (35-37). RAI ablation was also positively associated with GO activation (38-40). Factors associated with the relapse of GD, such as orbitopathy, smoking, free T4, thyrotropin-related antibodies had been reported in a meta-analysis (41).
Differences in ORT response rates with the same dose have been observed in different studies, possibly because of the differences in the time of administration of the intervention. Most studies have concluded the effects of GO treatment may be inversely associated with the duration of ophthalmopathy. A study reported that patients with GO who receive ORT within 2 years after symptom onset may have better overall results, especially when ORT is combined with corticosteroids (42). However, this window of treatment is likely even shorter (6 months) with ORT only. A prospective randomized double-blind trial of patients with moderate GO administered ORT of 20 Gy; the study demonstrated no difference in the treatment effect on proptosis from that of the natural course. However, the cutoff value for symptom-to-ORT duration, 1.3 years, was slightly longer than that in our data, and fewer than 10% of patients received ORT within 6 months compared with 30% in our study (43). This may explain why ORT was not as effective in their study. Another study reported a lower dose of 12 Gy, compared with 16 and 20 Gy, might be sufficient to improve soft tissue signs (44). A review suggest that lower dose could be considered in young patients without orbital dysmotility, while minimize theoretical oncogenic risk (45). Therefore, although most contemporary studies have reported using 2 Gy per fraction of ORT for patients with GO, we chose 1 Gy per fraction for lower toxicity.
Low-dose radiotherapy (LDRT, defined as ≤1.0 Gy per fraction) has an immunomodulatory effect (46). LDRT induces transforming growth factor-ß1 and IL-10, leading to decreased E-selectin expression on epithelial cells, which eventually causes reduced adhesion of peripheral blood mononuclear cells, including T cells, B cells, and macrophages, to the endothelium (46). It also decreases the activity of stimulated macrophages in inflamed tissues by reducing the activity of inducible nitric oxide synthase, which decreases nitric oxide, thereby reducing oxidative stress to the tissues. Decreased TNF-α expression and increased IL-10 expression are also observed in peripheral blood mononuclear cells following LDRT (46). To summarize, compared with the most commonly used 1.8–2.0 Gy per fraction in the treatment of cancer, the 1-Gy dose likely yields not only fewer side effects but also more immunomodulatory effects on lymphocytes, macrophages, and inflammatory cells and is thus more effective for GO-related proptosis.
The reported side effects of ORT using intensity-modulated radiotherapy (IMRT) with 20 Gy in 10 fractions include eye redness (7.8%), sideburn hair loss (16.4%), and milphosis or madarosis (19.8%) (27). However, in our patient population, no side effects were observed. One study applied 20 Gy in 10 fractions using three-dimensional (3D) radiotherapy using opposing fields plus concomitant steroids. Acute toxicities of grade 1–2 keratitis were reported in 19.5% of patients, and grade 3 keratitis was reported in 2.4% of the patients; late cataracts were reported in 7.4% of patients at a median follow-up time of 24 months (47). Use of 3D conformal radiotherapy (3D-CRT) with opposing fields led to RT-induced conjunctivitis in 18% of patients receiving 10 Gy in 10 fractions over 2 weeks, 36% of patients receiving 20 Gy in 10 fractions over 2 weeks, and of no patients receiving 20 Gy in 20 fractions over 20 weeks (22). Because of the planning limitations of 3D-CRT, hot spots of the radiotherapeutic dose may be present; dose homogeneity has since been improved with IMRT and further improved with VMAT, which is the technique used in our study (48,49). The recommended dose of radiation based on normal tissue tolerance administered at 2 Gy per fraction is ≤10 Gy, <50 Gy, and <55 Gy for the lens, retina, and optic nerve, respectively, with complication rates of 5% for cataracts, <1% for retinopathy, and <3% for optic neuropathy (50). In our study, the dose was considerably lower—half the fraction dose (1 Gy), which may explain the lack of side effects or complications, including no corneal erosion or retinopathy, in our study; moreover, the VMAT technique may have allowed the administration of an optimized homogenous low dose to the cornea and lens.
The limitations of this study are its retrospective and nonrandomized character. Prospective randomized studies should be conducted to validate our findings. Also, the follow-up time could be longer to observe late recurrence. Although 10 Gy in 10 fractions was shown to be effective for GO, a boost dose depending on GO severity or in patients with relapse, if any, may be considered in the future—a reduced-field boost to the site of gross disease is common in cancer treatment. Furthermore, targeting the application of ORT to only the affected eye in recurrent cases may also be an option to prevent administration of unnecessary doses to the unaffected eye. Moreover, studies of concurrent steroids and ORT are ongoing in our institute. Future studies should evaluate the combination of ORT with concomitant steroid or promising biologics with known immunopathological implications for GO, including mycophenolate mofetil (51), methotrexate (52), cyclosporine (53), TNF-α blockers [adalimumab (54), etanercept (55)], IL-6 receptor antagonists (tocilizumab) (56), B-cell depleting agents (rituximab) (55), and anti-insulin-like growth factor-1 receptor agents (teprotumumab) (57). In addition, in patients refractory to low-dose ORT and requiring surgery, pathological analysis of the retro-orbital tissues could yield a better understanding of the possible methods for the improved treatment of this disease. Finally, personalizing ORT according to EUGOGO and CAS may also be a direction for further research.
Conclusions
In our retrospective cohort study, ORT with 10 fractions of 1 Gy daily was found to be a safe and effective alternative for proptosis in patients with moderate to severe GO. ORT alone led to modest proptosis regression during the 4-year follow-up. LDRT may be especially beneficial to patients receiving treatment within 6 months of proptosis, in a euthyroid state, with female sex, or with prominent EOM enlargement on CT images. LDRT not only reduces the visual complications but remains effective. Thus, ORT with 10 Gy in 10 fractions applied at an early state of GO, in addition to corticosteroids, surgery, or other targeted biologics, may serve as a reasonable option for the treatment of proptosis in patients with moderate to severe GO. To achieve the best possible results with the lowest complication rates, use of a multidisciplinary team, including endocrinologists, general surgeons, ophthalmologists, and radiation oncologists, is crucial. Based on our results, LDRT may be considered a treatment option for moderate to severe GO and proptosis within 6 months.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/tro-21-10
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tro-21-10). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Shin Kong Wu Ho-Su Memorial Hospital (20190407R) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dickinson AJ, Perros P. Controversies in the clinical evaluation of active thyroid-associated orbitopathy: use of a detailed protocol with comparative photographs for objective assessment. Clin Endocrinol (Oxf) 2001;55:283-303. [Crossref] [PubMed]
- Otto EA, Ochs K, Hansen C, et al. Orbital tissue-derived T lymphocytes from patients with Graves' ophthalmopathy recognize autologous orbital antigens. J Clin Endocrinol Metab 1996;81:3045-50. [PubMed]
- Bahn RS. Graves' ophthalmopathy. N Engl J Med 2010;362:726-38. [Crossref] [PubMed]
- Mourits MP, Koornneef L, Wiersinga WM, et al. Clinical criteria for the assessment of disease activity in Graves' ophthalmopathy: a novel approach. Br J Ophthalmol 1989;73:639-44. [Crossref] [PubMed]
- Werner SC. Modification of the classification of the eye changes of Graves' disease: recommendations of the Ad Hoc Committee of the American Thyroid Association. J Clin Endocrinol Metab 1977;44:203-4. [Crossref] [PubMed]
- Bartalena L, Baldeschi L, Dickinson A, et al. Consensus statement of the European Group on Graves' orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol 2008;158:273-85. [Crossref] [PubMed]
- Dolman PJ, Rootman J. VISA Classification for Graves orbitopathy. Ophthalmic Plast Reconstr Surg 2006;22:319-24. [Crossref] [PubMed]
- Douglas RS, Gupta S. The pathophysiology of thyroid eye disease: implications for immunotherapy. Curr Opin Ophthalmol 2011;22:385-90. [Crossref] [PubMed]
- Thomas HM, Jr WA. Progressive exophthalmus following thyroidectomy. Bull Johns Hopkins Hosp 1936;99-113.
- Rajendram R, Bunce C, Lee RW, et al. Orbital radiotherapy for adult thyroid eye disease. Cochrane Database Syst Rev 2012;CD007114 [Crossref] [PubMed]
- Marcocci C, Bartalena L, Bogazzi F, et al. Role of orbital radiotherapy in the treatment of Graves' ophthalmopathy. Exp Clin Endocrinol 1991;97:332-7. [Crossref] [PubMed]
- Teng CS, Crombie AL, Hall R, et al. An evaluation of supervoltage orbital irradiation for Graves' ophthalmopathy. Clin Endocrinol (Oxf) 1980;13:545-51. [Crossref] [PubMed]
- Brennan MW, Leone CR Jr, Janaki L. Radiation therapy for Graves' disease. Am J Ophthalmol 1983;96:195-9. [Crossref] [PubMed]
- Olivotto IA, Ludgate CM, Allen LH, et al. Supervoltage radiotherapy for Graves' ophthalmopathy: CCABC technique and results. Int J Radiat Oncol Biol Phys 1985;11:2085-90. [Crossref] [PubMed]
- Konishi J, Iida Y, Kasagi K, et al. Clinical evaluation of radiotherapy for Graves' ophthalmopathy. Endocrinol Jpn 1986;33:637-44. [Crossref] [PubMed]
- Kulig G, Andrysiak-Mamos E, Sowińska-Przepiera E, et al. Quality of life assessment in patients with Graves' disease and progressive infiltrative ophthalmopathy during combined treatment with methylprednisolone and orbital radiotherapy. Endokrynol Pol 2009;60:158-65. [PubMed]
- Sandler HM, Rubenstein JH, Fowble BL, et al. Results of radiotherapy for thyroid ophthalmopathy. Int J Radiat Oncol Biol Phys 1989;17:823-7. [Crossref] [PubMed]
- Gorman CA, Garrity JA, Fatourechi V, et al. A Prospective, Randomized, Double-blind, Placebo-controlled Study of Orbital Radiotherapy for Graves' Ophthalmopathy. Ophthalmology 2020;127:S160-71. [Crossref] [PubMed]
- Petersen IA, Kriss JP, McDougall IR, et al. Prognostic factors in the radiotherapy of Graves' ophthalmopathy. Int J Radiat Oncol Biol Phys 1990;19:259-64. [Crossref] [PubMed]
- Fells P. Thyroid-associated eye disease: clinical management. Lancet 1991;338:29-32. [Crossref] [PubMed]
- Lloyd WC 3rd, Leone CR Jr. Supervoltage orbital radiotherapy in 36 cases of Graves' disease. Am J Ophthalmol 1992;113:374-80. [Crossref] [PubMed]
- Kahaly GJ, Rösler HP, Pitz S, et al. Low- versus high-dose radiotherapy for Graves' ophthalmopathy: a randomized, single blind trial. J Clin Endocrinol Metab 2000;85:102-8. [PubMed]
- Gerling J, Kommerell G, Henne K, et al. Retrobulbar irradiation for thyroid-associated orbitopathy: double-blind comparison between 2.4 and 16 Gy. Int J Radiat Oncol Biol Phys 2003;55:182-9. [Crossref] [PubMed]
- Hiromatsu Y, Yang D, Bednarczuk T, et al. Cytokine profiles in eye muscle tissue and orbital fat tissue from patients with thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 2000;85:1194-9. [PubMed]
- Hsu WC, Lui LT, Tseng S, et al. Radiotherapy in Graves Ophthalmopathy at National Taiwan University Hospital. Therapeutic Radiology and Oncology 1999;6:285-93.
- Grassi P, Strianese D, Piscopo R, et al. Radiotherapy for the treatment of thyroid eye disease-a prospective comparison: Is orbital radiotherapy a suitable alternative to steroids? Ir J Med Sci 2017;186:647-52. [Crossref] [PubMed]
- Li YJ, Luo Y, He WM, et al. Clinical outcomes of graves' ophthalmopathy treated with intensity modulated radiation therapy. Radiat Oncol 2017;12:171. [Crossref] [PubMed]
- Sisti E, Menconi F, Leo M, et al. Long-term outcome of Graves' orbitopathy following high-dose intravenous glucocorticoids and orbital radiotherapy. J Endocrinol Invest 2015;38:661-8. [Crossref] [PubMed]
- Tsai CC, Kau HC, Kao SC, et al. Exophthalmos of patients with Graves' disease in Chinese of Taiwan. Eye (Lond) 2006;20:569-73. [Crossref] [PubMed]
- Migliori ME, Gladstone GJ. Determination of the normal range of exophthalmometric values for black and white adults. Am J Ophthalmol 1984;98:438-42. [Crossref] [PubMed]
- Chang AA, Bank A, Francis IC, et al. Clinical exophthalmometry: a comparative study of the Luedde and Hertel exophthalmometers. Aust N Z J Ophthalmol 1995;23:315-8. [Crossref] [PubMed]
- Aniszewski JP, Valyasevi RW, Bahn RS. Relationship between disease duration and predominant orbital T cell subset in Graves' ophthalmopathy. J Clin Endocrinol Metab 2000;85:776-80. [PubMed]
- Kumar S, Bahn RS. Relative overexpression of macrophage-derived cytokines in orbital adipose tissue from patients with graves' ophthalmopathy. J Clin Endocrinol Metab 2003;88:4246-50. [Crossref] [PubMed]
- Feldon SE, O'loughlin CW, Ray DM, et al. Activated human T lymphocytes express cyclooxygenase-2 and produce proadipogenic prostaglandins that drive human orbital fibroblast differentiation to adipocytes. Am J Pathol 2006;169:1183-93. [Crossref] [PubMed]
- Kim SJ, Kim BJ, Lee HB, et al. Thyroid associated orbitopathy following periocular surgery. Korean J Ophthalmol 2006;20:82-6. [Crossref] [PubMed]
- Baldeschi L, Lupetti A, Vu P, et al. Reactivation of Graves' orbitopathy after rehabilitative orbital decompression. Ophthalmology 2007;114:1395-402. [Crossref] [PubMed]
- Xu L, Glass LR, Kazim M. Reactivation of thyroid eye disease following extraocular muscle surgery. Ophthalmic Plast Reconstr Surg 2014;30:e5-6. [Crossref] [PubMed]
- Acharya SH, Avenell A, Philip S, et al. Radioiodine therapy (RAI) for Graves' disease (GD) and the effect on ophthalmopathy: a systematic review. Clin Endocrinol (Oxf) 2008;69:943-50. [Crossref] [PubMed]
- Ponto KA, Zang S, Kahaly GJ. The tale of radioiodine and Graves' orbitopathy. Thyroid 2010;20:785-93. [Crossref] [PubMed]
- Vannucchi G, Campi I, Covelli D, et al. Graves' orbitopathy activation after radioactive iodine therapy with and without steroid prophylaxis. J Clin Endocrinol Metab 2009;94:3381-6. [Crossref] [PubMed]
- Struja T, Fehlberg H, Kutz A, et al. Can we predict relapse in Graves' disease? Results from a systematic review and meta-analysis. Eur J Endocrinol 2017;176:87-97. [Crossref] [PubMed]
- Bartalena L, Marcocci C, Chiovato L, et al. Orbital cobalt irradiation combined with systemic corticosteroids for Graves' ophthalmopathy: comparison with systemic corticosteroids alone. J Clin Endocrinol Metab 1983;56:1139-44. [Crossref] [PubMed]
- Gorman CA, Garrity JA, Fatourechi V, et al. A prospective, randomized, double-blind, placebo-controlled study of orbital radiotherapy for Graves' ophthalmopathy. Ophthalmology 2001;108:1523-34. [Crossref] [PubMed]
- Johnson KT, Wittig A, Loesch C, et al. A retrospective study on the efficacy of total absorbed orbital doses of 12, 16 and 20 Gy combined with systemic steroid treatment in patients with Graves' orbitopathy. Graefes Arch Clin Exp Ophthalmol 2010;248:103-9. [Crossref] [PubMed]
- Godfrey KJ, Kazim M. Radiotherapy for Active Thyroid Eye Disease. Ophthalmic Plast Reconstr Surg 2018;34:S98-S104. [Crossref] [PubMed]
- Rödel F, Keilholz L, Herrmann M, et al. Radiobiological mechanisms in inflammatory diseases of low-dose radiation therapy. Int J Radiat Biol 2007;83:357-66. [Crossref] [PubMed]
- Nicosia L, Reverberi C, Agolli L, et al. Orbital Radiotherapy Plus Concomitant Steroids in Moderate-to-Severe Graves' Ophthalmopathy: Good Results After Long-Term Follow-Up. Int J Endocrinol Metab 2019;17:e84427 [Crossref] [PubMed]
- Hong L, Hunt M, Chui C, et al. Intensity-modulated tangential beam irradiation of the intact breast. Int J Radiat Oncol Biol Phys 1999;44:1155-64. [Crossref] [PubMed]
- Zhang Q, Yu XL, Hu WG, et al. Dosimetric comparison for volumetric modulated arc therapy and intensity-modulated radiotherapy on the left-sided chest wall and internal mammary nodes irradiation in treating post-mastectomy breast cancer. Radiol Oncol 2015;49:91-8. [Crossref] [PubMed]
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109-22. [Crossref] [PubMed]
- Ye X, Bo X, Hu X, et al. Efficacy and safety of mycophenolate mofetil in patients with active moderate-to-severe Graves' orbitopathy. Clin Endocrinol (Oxf) 2017;86:247-55. [Crossref] [PubMed]
- Strianese D, Iuliano A, Ferrara M, et al. Methotrexate for the treatment of thyroid eye disease. J Ophthalmol 2014;2014:128903 [Crossref] [PubMed]
- Witte A, Landgraf R, Markl A, et al. Treatment of Graves' ophthalmopathy with cyclosporin A. Klin Wochenschr 1985;63:1000-4. [Crossref] [PubMed]
- Ayabe R, Rootman DB, Hwang CJ, et al. Adalimumab as steroid-sparing treatment of inflammatory-stage thyroid eye disease. Ophthalmic Plast Reconstr Surg 2014;30:415-9. [Crossref] [PubMed]
- Boskovic O, Medenica S, Radojevic N, et al. Etanercept in the treatment of Graves' ophthalmopathy with primary hypothyroidism and rheumatoid arthritis. Cent Eur J Immunol 2019;44:463-5. [Crossref] [PubMed]
- Hamed Azzam S, Kang S, Salvi M, et al. Tocilizumab for thyroid eye disease. Cochrane Database Syst Rev 2018;11:CD012984 [PubMed]
- Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for Thyroid-Associated Ophthalmopathy. N Engl J Med 2017;376:1748-61. [Crossref] [PubMed]
Cite this article as: Ding BY, Bai CH, Chi KH, Shih MJ, Ko HL. Orbital radiotherapy for proptosis in patients with Graves’ ophthalmopathy. Ther Radiol Oncol 2021;5:15.


