Post-mastectomy radiotherapy between implant-based reconstruction and autologous flap reconstruction
Introduction
Although breast conservation plus radiation had equivalent outcome to mastectomy after 1980s (1), mastectomy still remind one of the surgical choice of breast cancer. Radiation therapy (RT) is an increasingly common adjuvant therapy in breast cancer which provides loco-regional control and improved survival in selected patient (2,3). Furthermore, breast reconstruction has also become an integrated part of breast cancer treatment due to long-term psychosexual health factors and its importance for breast cancer survivors (4). Both autogenous flap and implant-based reconstruction provides satisfactory reconstructive options, although each has its own advantages and disadvantages. In recent years, implant reconstruction rates in the United States have risen steadily in the irradiated patient population from 27% to 52%, with a concomitant decrease in autologous reconstruction, from 56% to 32%, over the past decade (5).
Nevertheless, reconstruction may cause surgery complications, such as infection, capsular tightening, implant rupture, flap necrosis and reconstruction failure, from 3% to 16% compared to patients who do not received reconstruction. Moreover, post-mastectomy irradiation increased complications two- to three-fold in who did immediate breast reconstruction, from 16% to 42%. Both post-mastectomy irradiation and breast reconstruction were strong independent predictors of complications (6).
This research analyzed the difference of surgical complication, quality of life and satisfaction between autogenous and implant-based breast reconstruction, and the difference of dose dosimetry and irradiation complication in adjuvant radiation. These may help us make decision of the choice of reconstruction type and discuss with the patient about the effect of radiation may have on her reconstruction outcome.
Methods
This research included 15 years (between January 2003 and December 2017) of female breast cancer patient who received mastectomy with breast reconstruction and adjuvant RT to ipsilateral chest wall +/− regional lymph nodes in our hospital. Basic data, date of breast cancer surgery, tumor characteristics (laterality, size and lymph nodes), pathology report and planned treatment characteristics (radiotherapy, chemotherapy, endocrine therapy, and immunotherapy) were collected from the medical records. We exclude the patients who had distant metastasis, and who didn’t received standard adjuvant RT (Radiotherapy to other than ipsilateral chest wall or chest wall plus lymph nodes target area), and no regularly follow up patients, and no dosimetric data available in the verification system, and less than one year follow up time which may mask the late complication. We survey the type of reconstruction (autogenous, implant-based breast reconstruction or a concurrent combination of both tissue and implant reconstruction), the timing of reconstruction (immediate, two-stage with tissue expander, delayed reconstruction) and post operation early or late complication.
We collect these reconstruction patients who had received adjuvant radiation and analyze dose dosimetry and treatment technique: dates of radiotherapy (start and end), target volume (chest wall or chest wall plus lymph nodes), total dose, number of fractions, boost and bolus (if any), were retrieved from the RT charts. All patients need to receive full course of RT. Dose dosimetry analysis included planning target volume (PTV) D95 (dosage of target irradiated with 95% of isodose), maximum dose, CTV V105 (volume irradiated with 105% of isodose), CTV V107 (volume irradiated with 107% of isodose), ipsilateral lung V20 (volume of lung exposed to 20 Gy or more), ipsilateral lung V5 (volume of lung exposed to 5 Gy or more), mean heart dose and heart V25 (volume of heart exposed to 25 Gy or more).
Two-sample t-test was used to compare differences in continuous variables in the 2 groups. Difference between categorical variables was performed using the Chi-squared test. Results were considered significant at a P value of less than 0.05.
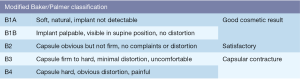
Patients were routinely followed up every 3 months for at least one year. Acute and late toxicities associated to RT were recorded according to the Common Terminology Criteria for Adverse Events version 3.0. Capsular contracture was classified via modified Baker/Palmer classification (Figure 1).
This study was approved by the Institutional Review Board of China Medical University Hospital (CMUH107-REC3-044).
Results
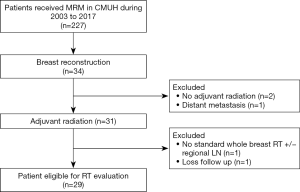
During January 2003 to December 2017, there were 227 patients received mastectomy. Among these patients, 34 patients performed breast reconstruction. After excluded the patients who didn’t meet the inclusion criteria, 29 patients were enrolled in this retrospective study (Figure 2).
The median follow-up time was 40 months (range from 12 to 119 months). The patients’ characteristics are shown in Table 1. The timing of reconstruction included 25 patients underwent immediate reconstructions and 4 people underwent two-staged reconstructions. No delayed reconstruction had performed. Reconstruction method of implant-base reconstruction included 14 for silicone, 1 for saline and 4 for tissue expander reconstructions. In autologous reconstruction group, 7 patients underwent transverse rectus abdominis myocutaneous flap (TRAM), and 2 for deep inferior epigastric perforator flap (DIEP).
Table 1
| Characteristics | Implant | Autologous flap |
|---|---|---|
| No. of patients | 19 | 10 |
| Age | ||
| Median [range] | 53 [20–74] | 48 [32–71] |
| p-stage | ||
| I | 1 | 1 |
| II | 3 | 4 |
| III | 15 | 5 |
| Margin | ||
| free | 14 (73.7%) | 6 (60%) |
| involved | 5 (26.3%) | 4 (40%) |
| Receptor status | ||
| Luminal | 14 | 8 |
| Her-2 | 1 | 1 |
| TNBC | 4 | 1 |
| Timing of reconstruction | ||
| Immediate | 15 | 10 |
| Stage | 4 | 0 |
| Delay | 0 | 0 |
| Reconstruction method | Silicone: 14 | TRAM: 7 |
| Saline: 1 | DIEP: 2 | |
| Tissue expander: 4 | Others: 1 | |
| Chemotherapy | ||
| All | 16 | 8 |
| Neoadjuvant | 5 | 2 |
| Adjuvant | 13 | 7 |
| Hormone therapy | ||
| Neoadjuvant | 1 | 3 |
| Adjuvant | 17 | 9 |
TRAM, transverse rectus abdominis myocutaneous flap; DIEP, deep inferior epigastric perforator flap.
The overall post-operative complications were shown in Table 2. Post-operative early complication including infection, poor wound healing and reconstructive failure, all developed before irradiation in 5 patients, 3 implant (15.8%) and 2 autologous flap (20%) reconstruction. Two (10.5%) out of the 3 implant reconstruction patients, both of them use tissue expander for stage reconstruction, received revision operation. One for debridement and the other need to shift implant to TRAM reconstruction due to reconstruction failure. The 2 autologous flap patients (20%) suffered from poor wound healing and underwent debridement.
Table 2
| Complications | Implant | Autologous flap |
|---|---|---|
| Overall postoperative complications | 11 (57.9%) | 6 (60%) |
| Postoperative early complications | 3 (15.8%) | 2 (20%) |
| Infection | 3 | 0 |
| Poor wound healed | 2 | 2 |
| Revision surgery due to early complications | 2 (10.5%) | 2 (20%) |
| Debridement | 1 | 2 |
| Failure | 1 | 0 |
| Late complications | 9 (47.4%) | 6 (60%) |
| Capsular contracture | 5 | 0 |
| Skin contracture | 2 | 1 |
| Fat necrosis | 1 | 3 |
| Skin necrosis | 0 | 1 |
| Hematoma | 1 | 0 |
| Failure | 1 | 0 |
| Abdominal hernia | 0 | 1 |
| Revision surgery due to late complications | 3 (15.8%) | 3 (30%) |
| Capsular contracture | 1 | 0 |
| Skin contracture | 1 | 2 |
| Failure | 1 | 0 |
| Abdominal hernia | 0 | 1 |
Late complications consist of capsular contracture, skin contracture, skin necrosis, fat necrosis, abdominal hernia, and reconstructive failure which were shown in Table 2. Nine (47.4%) patients in implant reconstruction and 6 (60%) patients in autologous flap reconstruction suffered from late complication. There were 3 patients in each group needed further revision operation due to severe contracture, repair or reconstruction failure.
Radiation technique was shown in Table 3. Fifteen patients had received tumor bed electron boost for additional 10 to 16 Gy, only 7 of them had positive margin. None of these 29 patients added bolus during whole breast irradiation. Lymph node irradiation technique was 12 people via anterior-posterior field, and 15 people via IMRT. Acute radiation complication of grade 3 skin reaction only developed in one person with implant reconstruction. No grade 2 or above RT late complication developed.
Table 3
| RT technique | Implant | Autologous flap |
|---|---|---|
| N | 19 | 10 |
| Boost | 10 | 5 |
| Margin (+) | 3 | 4 |
| Margin (−) | 7 | 1 |
| Margin (+) | 5 | 4 |
| Boost | 3 | 4 |
| No boost | 2 | 0 |
| Bolus use | 0 | 0 |
| LN irradiation | ||
| AP field | 7 | 5 |
| IMRT | 10 | 5 |
| No including | 2 | 0 |
AP, antero-posterior; IMRT, intensity-modulated radiotherapy.
Dose dosimetry analysis included PTV D95, maximum dose, CTV V105, CTV V107, ipsilateral lung V20, ipsilateral lung V5, mean heart dose and heart V25 was shown in Table 4. Between implant and autologous flap reconstruction, there were no significant differences, no matter right side or left side breast cancer. Besides, both types of reconstruction techniques can reach acceptable dose distribution. Comparing patients who had revision of operation due to late complications which may be associated with RT, there was still no significant difference between each other except ipsilateral lung V5 and V20 (Table 5).
Table 4
| Dosimetry parameter | Implant (n=19) | Autologous flap (n=10) | P value | |||
|---|---|---|---|---|---|---|
| Median | Range | Median | Range | |||
| PTV D95 (%) | 95.95 | 91.8–99.1 | 96 | 92.4–98.1 | 0.52 | |
| Max (%) | 109.7 | 101.9–114.4 | 112 | 105–115.3 | 0.06 | |
| CTV V105 (%) | 18.1 | 4.1–66.1 | 21.7 | 8.0–55.0 | 0.93 | |
| CTV V105 (cm3) | 151.7 | 24.9–298.4 | 108.1 | 23.3–318.2 | 0.96 | |
| CTV V107 (%) | 0.8 | 0–17.0 | 4.0 | 0–30.1 | 0.24 | |
| CTV V107 (cm3) | 5.4 | 0–82.9 | 23.1 | 0.18–202 | 0.18 | |
| Ipsilateral lung, V20 (%) | 20.6 | 10.7–32.2 | 21.6 | 15.7–43.4 | 0.30 | |
| Ipsilateral lung, V5 (%) | 40.9 | 19.5–58.2 | 42.8 | 25.3–61.6 | 0.60 | |
| Mean heart (cGy) | 138 | 54–612 | 115 | 48–817 | 0.99 | |
| Heart V25 (%) | 0 | 0–6.7 | 0 | 0–10.8 | 0.97 | |
Table 5
| Dosimetry parameter | With complication (n=5) | Without complication (n=24) | P value | |||
|---|---|---|---|---|---|---|
| Median | Range | Median | Range | |||
| PTV D95 (%) | 95.9 | 91.8–98.4 | 97 | 92.8–99.1 | 0.67 | |
| Max (%) | 110.2 | 105–115.3 | 109.7 | 101.9–115.3 | 0.49 | |
| CTV V105 (%) | 19.1 | 2.7–66.1 | 19.6 | 8.1–24.1 | 0.32 | |
| CTV V105 (cm3) | 126.6 | 24.9–369 | 184.7 | 23.3–288.2 | 0.95 | |
| CTV V107 (%) | 1.3 | 0–30.1 | 2.6 | 0–6.2 | 0.49 | |
| CTV V107 (cm3) | 8.2 | 0–202 | 10.3 | 0.1–61.7 | 0.64 | |
| Ipsilateral lung, V20 (%) | 20.6 | 10.7–35.6 | 23.8 | 18–43.4 | 0.04 | |
| Ipsilateral lung, V5 (%) | 40.3 | 19.5–61.6 | 56.2 | 35.4–59.8 | 0.02 | |
| Mean heart (cGy) | 123 | 48–612 | 387 | 91–817 | 0.26 | |
| Heart V25 (%) | 0 | 0–6.7 | 5 | 0–10.8 | 0.17 | |
Discussion
Overall complication rates were more than double in the irradiation group compared with the nonirradiated group (7). Postoperative complications in patients who underwent implant reconstruction with variations in the timing of reconstruction were around 44–51%, which including prosthesis failure (27–37%), capsular contracture (8–13%), infection (7–11%), wound complications (6–8%) and hematoma (0–3%). In autologous reconstruction, the overall postoperative complications were around 27–37%, including autologous failure (3–5%), infection (6–9%), wound complication (10–11%), fat necrosis (6–11%) and hematoma (2–5%) (6). Overall postoperative complications were higher in patients with implant reconstruction compared with autologous reconstruction (45.3% versus 30.8%; P<0.001) (8). In our study, although small population, overall postoperative complications were around 60% in both groups of reconstruction which is comparable to than other study (42–68%) (6,7). Complications which need revision of operation were two times higher in autologous reconstruction group in our study, which is around 50% versus 26%. All of the early complications developed before irradiation. Severe late complications, which may be associated with RT and underwent second operation, developed 16% in implant reconstruction versus 20% in autologous reconstruction.
In our study, all the 4 patients who use tissue expender suffered from severe early or/and late complications and 3 of them need second operation due to reconstruction failure, poor wound healing and capsular contracture. Only one of them succeeded to exchange to silicone implant. In meta-analysis of implant-based breast reconstruction, radiation to tissue expender which cause reconstruction failure was significant more than radiation to permanent implant (20% versus 13.4%, P<0.001) due to overlying skin become thin and damaged leading to extrusion and increase tension (9). Whereas other smaller prospective study revealed similar complication rates observed between patients with tissue expanders versus permanent implants. Tissue expender may suit for the patient who was unsure for post mastectomy RT at the time of mastectomy (10). If adjuvant RT didn’t require, she can subsequently exchange for a permanent implant or autologous reconstruction. If adjuvant RT require, after chemotherapy and RT completed, she can remove tissue expender to permanent reconstruction (11).
Dose distribution and organ at risk analysis were also no significant in some paper (12,13). Adequate dose to the reconstructed breast is feasible in women who undergo immediate reconstruction after mastectomy, regardless of the reconstruction type or laterality of the treatment plan. Even though inclusion of IMN may result in higher doses to the heart, study suggest that clinically acceptable doses to the heart and lungs can be achieved in most patients (13). Besides, inclusion of the IMN as part of regional nodal irradiation can reduce the risks of local-regional failure and breast cancer mortality for patients with T1-2 breast cancer with one to three positive axillary nodes (3). There were five patients had IMN irradiation in our study, all of them achieved acceptable coverage. The maximum dose to the skin (58.5 versus 61.7 Gy; P=0.05) and the maximum dose to 1 cc of skin (54.4 versus 57.4 Gy; P=0.01) were associated with increased complication rates and should be given consideration as a vital organ. However, no specific cut off dosage had suggested (14).
Conclusions
Mastectomy with breast reconstructions and adjuvant radiotherapy are linked to high complication rates. Acceptable dose distribution to the reconstructed breast and organ at risk is feasible. Besides, there were also no significant difference of distribution between implant reconstruction and autologous reconstructions. The incidence of complications has no relevance to dose distribution.
Acknowledgments
The authors thank the members of the Cancer Center (China Medical University Hospital) for their invaluable help.
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2020.01.01). JAL serves as the unpaid editorial board members of Therapeutic Radiology and Oncology from Oct 2017 to Sep 2019. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of China Medical University Hospital (CMUH107-REC3-044). Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fisher B, Montague E, Redmond C, et al. Findings from NSABP Protocol No. B-04-comparison of radical mastectomy with alternative treatments for primary breast cancer. I. Radiation compliance and its relation to treatment outcome. Cancer 1980;46:1-13. [Crossref] [PubMed]
- McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127-35. [Crossref] [PubMed]
- Recht A, Comen EA, Fine RE, et al. Postmastectomy Radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. Pract Radiat Oncol 2016;6:e219-34. [Crossref] [PubMed]
- Cohen WA, Mundy LR, Ballard TN, et al. The BREAST-Q in surgical research: a review of the literature 2009–2015. Journal of Plastic, Reconstructive & Aesthetic Surgery 2016;69:149-62. [Crossref] [PubMed]
- Agarwal S, Kidwell KM, Farberg A, et al. Immediate Reconstruction of the Radiated Breast: Recent Trends Contrary to Traditional Standards. Ann Surg Oncol 2015;22:2551-9. [Crossref] [PubMed]
- Christante D, Pommier SJ, Diggs BS, et al. Using complications associated with postmastectomy radiation and immediate breast reconstruction to improve surgical decision making. Arch Surg 2010;145:873-8. [Crossref] [PubMed]
- Krueger EA, Wilkins EG, Strawderman M, et al. Complications and patient satisfaction following expander/implant breast reconstruction with and without radiotherapy. Int J Radiat Oncol Biol Phys 2001;49:713-21. [Crossref] [PubMed]
- Chetta MD, Aliu O, Zhong L, et al. Reconstruction of the Irradiated Breast: A National Claims-Based Assessment of Postoperative Morbidity. Plast Reconstr Surg 2017;139:783-92. [Crossref] [PubMed]
- Ricci JA, Epstein S, Momoh AO, et al. A meta-analysis of implant-based breast reconstruction and timing of adjuvant radiation therapy. J Surg Res 2017;218:108-16. [Crossref] [PubMed]
- Santosa KB, Chen X, Qi J, et al. Postmastectomy Radiation Therapy and Two-Stage Implant-Based Breast Reconstruction: Is There a Better Time to Irradiate? Plast Reconstr Surg 2016;138:761-9. [Crossref] [PubMed]
- Poppe MM, Agarwal JP. Breast Reconstruction With Postmastectomy Radiation: Choices and Tradeoffs. J Clin Oncol 2017;35:2467-70. [Crossref] [PubMed]
- Liljegren A, Unukovych D, Gagliardi G, et al. No difference in dose distribution in organs at risk in postmastectomy radiotherapy with or without breast implant reconstruction. Radiat Oncol 2014;9:14. [Crossref] [PubMed]
- Chung E, Marsh RB, Griffith KA, et al. Quantifying dose to the reconstructed breast: can we adequately treat? Med Dosim 2013;38:55-9. [Crossref] [PubMed]
- Muresan H, Lam G, Cooper BT, et al. Impact of Evolving Radiation Therapy Techniques on Implant-Based Breast Reconstruction. Plast Reconstr Surg 2017;139:1232e-9e. [Crossref] [PubMed]
Cite this article as: Cheng HY, Liang JA. Post-mastectomy radiotherapy between implant-based reconstruction and autologous flap reconstruction. Ther Radiol Oncol 2020;4:1.