Combined stereotactic body radiotherapy with conventionally fractionated radiotherapy successfully managing a locoregionally advanced lung cancer patient: a case report
Introduction
An estimated 234,030 new cases of lung cancer were expected in the United States in 2018 (1). About 80% to 85% cases comprise non-small cell lung cancer (NSCLC). At diagnosis, cases of locoregionally advanced cancer accounted about 27.6% (2).
In patients with locoregionally advanced NSCLC, concurrent chemoradiotherapy (CCRT) was the preferred treatment in cases where surgery could not be performed. Despite improvement in radiotherapy (RT) techniques and systemic therapies, the overall survival, disease-free survival, and local control remain unsatisfactory among these patients. For example, use of RTOG 06-17 escalated the total dose of conventional RT required but failed to improve local control and patient survival (3).
Recently, stereotactic body radiation therapy (SBRT) reportedly demonstrated satisfactory local control of cancer in patients with early-stage NSCLC. Both local control rates and overall survival improved with a biologically effective dose (BED) of ≥100 Gy compared to a BED <100 Gy (4). Moreover, three prospective studies demonstrated promising results (76% to 86%) of one-year local control of lung cancer after lung SBRT, including the improvement of isolated residual tumor from patients with locoregionally advanced NSCLC (5-7). A phase I study revealed that SBRT combined with concurrent conventionally fractionated chemoradiation passed the dose constraint criteria of normal tissues (8). However, the effectiveness of SBRT in patients with locoregionally advanced lung cancer remains unclear.
Herein, we report the case of a patient with locoregionally advanced NSCLC. Combination of SBRT (60 Gy, 3 fractions) and conventionally fractionated RT (62 Gy, 31 fractions) were administered to the primary tumor and the mediastinal lymph nodes, respectively.
We present the following case in accordance with the CARE Guideline.
Case presentation
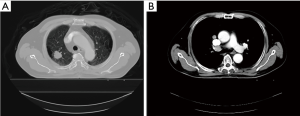
A 55-year-old heavy smoking man was diagnosed with adenocarcinoma of the right upper lung lobe [cT1bN2M0, stage IIIA; (Aug., 2015; AJCC 7th edition)] with a wild-type epithelial growth factor receptor (Figure 1). The patient had severe chronic obstructive pulmonary disease and demonstrated poor outcome after he underwent a total laminectomy, 1.5 months before diagnosis, at the C6-T4 level for the removal of dural arteriovenous fistula. He had paraplegia, with bilateral weakness of hands and choked easily. Because of his significant co-morbidity, the surgery was expected to involve high risk; thus, the surgical oncologist of the thoracic medicine department recommended against the surgery. Therefore, treatment with definitive CCRT was suggested after discussion with the patient and his family.
Because of his poor medical condition, preservation of pulmonary function assumed priority. To achieve maximal tumor control of both local and regional diseases, and to prevent additional toxicities, SBRT with conventionally fractionated RT seemed a better alternative. Therefore, we performed a combination of RT techniques such as: SBRT for eradicating the primary lung tumor and conventionally fractionated RT for managing the mediastinal nodal disease.
In the RT treatment plan, SBRT, with a total dose of 60 Gy in 3 fractions, was prescribed to treat the primary lung tumor. By observing the internal motion with respiration-gated simulation technique, the primary plan target volume (PTV) was generated from the gross lung tumor with a circumferential margin of 5 mm and a longitudinal margin of 10 mm (Figure 2A). The radiation dose was prescribed to cover >95% of the primary PTV, and >99% of the primary PTV received a minimum of 90% of the prescribed dose (i.e., 54 Gy; Figure 2A,B). Conventionally fractionated RT was administered to the gross mediastinal nodal disease with a total dose of 62 Gy in 31 fractions. Nodal PTV was generated from the gross nodal disease with a margin of 5–10 mm [i.e., nodal clinical target volume (CTV)] and a set-up margin of 5 mm (Figure 2B). The radiation dose was prescribed on the 95% isodose line with a coverage rate of >97% of the nodal PTV (Figure 2A,B). Both SBRT and conventionally fractionated RT were delivered with volumetric-modulated arc therapy technique. The combined SBRT and conventionally fractionated RT achieved an overall clinically acceptably physical profile on normal organs (Table 1), as reported by the RTOG 06-17 (3) and RTOG 06-18 trials (9).
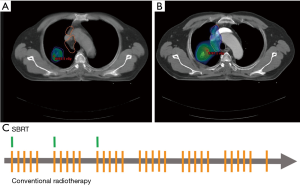
Table 1
| Critical structures | Combined RT plan | SBRT-alone plan |
|---|---|---|
| Spinal Cord | Max. =22.4 Gy | Max. =11.2 Gy |
| Whole Lung (Right & Left) | V20 =14.3% | |
| Mean =10.7 Gy | ||
| Esophagus | V60 =0% | Max. =11.8 Gy |
| Mean =13.7 Gy | ||
| Ipsilateral Brachial Plexus | Max. =2.4 Gy | Max. =1.2 Gy |
| Median =1.2 Gy | ||
| Mean =1.6 Gy | ||
| Heart/Pericardium | V60 =0% | Max. =7.0 Gy |
| V45 <1% | ||
| V40 <1% | ||
| Mean =2.2 Gy | ||
| Trachea and Ipsilateral Bronchus | Max. =14.5 Gy | |
| Skin | Max. =6.8 Gy |
RT, radiotherapy; SBRT, stereotactic body radiotherapy.
From October 2015 to November 2015, a total dose of 62 Gy of conventionally fractionated RT was delivered successfully in 31 fractions (once every week days for 6 weeks). Concurrently, a total dose of 60 Gy of SBRT was delivered in 3 fractions (once per week, during the first 3 weeks of the entire course of RT) (Figure 2C). During the RT course, respiration-gated technique, daily on-board images, and weekly cone-beam computed tomography (CT) were performed to assist precise irradiation. Concurrently, chemotherapy was administered every 3 weeks with a combined regimen of vinorelbine (60 mg/m2) and cisplatin (60 mg/m2).
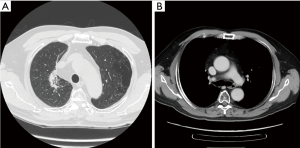
During the whole treatment course of CCRT, no significant acute toxicities or late sequelae occurred until the last follow-up (i.e., 2 years after CCRT course). Only minimal toxicities, such as grade 1 fatigue and grade 1 poor appetite, were observed, according to the Common Toxicity Criteria of Adverse Events v4.0 (10). A durable complete response of the primary lung tumor and regional nodal disease was confirmed at 6 months, 1 year, and 2.5 years after RT (Figure 3).
The patient stated that he tolerated well during the whole course of the treatment and that no specific symptoms were noted during the following time.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Discussion
Even today, patients with locoregionally advanced lung cancer still demonstrate unsatisfactory clinical outcomes. A systematic review showed high locoregional failure rates of 31–100% after CCRT regimen (11). According to the RTOG 06-17 trial, dose escalation of RT to 74 Gy failed to improve the overall survival and tumor control when compared with the initial dose of 60 Gy (3). The 2-year local failure rate was 30.7% for the 60-Gy arm and 38.6% for the 74-Gy arm.
In patients with early-stage NSCLC who were medically inoperable, several prospective clinical trials have demonstrated that SBRT improves local control (~85%) and overall survival (~60%) at 3 years post therapy (12-17). TROG09.02 (CHISEL), a randomized trial that compared the efficacy of SBRT with conventional RT to manage patients with inoperable stage I NSCLC, reported significant improvements of overall survival [hazard ratio (HR), 0.51; 95% CI, 0.51–0.911; P<0.02] and absence of local failure (HR, 0.29; 95% CI, 0.130–0.662; P<0.002) (18). Irrespective of the biological mechanism, information of the optimized total dose and fractional size of SBRT remains unclear. Onishi et al., reported that the rates of local control and 3-year overall survival were 91.9% and 88.4%, respectively, for a BED of ≥100 Gy compared with 73.6% and 69.4%, respectively, for a BED of <100 Gy in patients with early-stage NSCLC (4).
To improve local control, SBRT, as an adjunct for treating residual disease, was adopted by three prospective studies for locally advanced NSCLC. The 1-year local control rates in these studies ranged from 76% to 86% with a prescribed a BED of 100–150 Gy (5-7). Higgins et al., reported a maximally tolerated dose of 30 Gy in 5 fractions as an adjunct to 44 Gy of thoracic CCRT for patients with locally advanced NSCLC, with a total BED of 100.8 Gy (7).
Studies that have combined the two RT techniques to achieve locoregional irradiation of a large area for gaining twice the benefits are not uncommon. For example, in patients with head and neck cancer, split-field intensity-modulated radiation therapy (IMRT) has been reported to be comparable to whole-field IMRT (19). Two benefits of split-field IMRT have been documented—first, the application of IMRT to primary tumors and upper nodal disease provides dose-focused and normal-tissue-sparing effects of IMRT, and second, using AP-PA half-beam central-blocked irradiation to the lower neck helps reduce the dose administered to the larynx/hypopharynx to preserve speech and swallowing functions. Herein, our case suggested a new technical combination of RT to gain twice the benefits of SBRT for the improvement of primary lung tumor, and conventionally fractionated RT to treat mediastinal nodal disease.
Till date, SBRT has been proven to be a curative modality for treating patients with early-stage NSCLC. A phase I study showed that combination of SBRT with concurrent administration of conventionally fractionated chemoradiation could pass the normal tissue dose constraints in patients with stage III NSCLC; however, the primary outcome of toxicities had not been published yet (8). The efficacy of SBRT to manage locoregionally advanced lung cancer remains unclear. Therefore, more data are required for conclusive results. To our best knowledge, the present study has strived to discover a new treatment modality using combined CCRT with concurrent SBRT and conventional RT for managing locoregionally advanced NSCLC. Durable tumor control with minimal toxicities could be achieved. Therefore, additional prospective clinical trials are encouraged to obtain conclusive results.
Concurrent administration of SBRT in conjunction with conventionally fractionated RT may be effective for the total management of both primary tumor and nodal disease in patients with locoregionally advanced NSCLC. Favorable treatment outcomes with minimal toxicities could be achieved with this strategy. However, additional randomized control trials should be undertaken to distinctly demarcate the actual effect size in this type of technical combination of RT.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2019.11.03). SKH serve as an unpaid editorial board members of Therapeutic Radiology and Oncology from Oct 2017 to Sep 2019. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Morgensztern D, Ng SH, Gao F, Govindan R. Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol 2010;5:29-33. [Crossref] [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. [Crossref] [PubMed]
- Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004;101:1623-31. [Crossref] [PubMed]
- Feddock J, Arnold SM, Shelton BJ, et al. Stereotactic body radiation therapy can be used safely to boost residual disease in locally advanced non-small cell lung cancer: a prospective study. Int J Radiat Oncol Biol Phys 2013;85:1325-31. [Crossref] [PubMed]
- Hepel JT, Leonard KL, Safran H, et al. Stereotactic Body Radiation Therapy Boost After Concurrent Chemoradiation for Locally Advanced Non-Small Cell Lung Cancer: A Phase 1 Dose Escalation Study. Int J Radiat Oncol Biol Phys 2016;96:1021-7. [Crossref] [PubMed]
- Higgins KA, Pillai RN, Chen Z, et al. Concomitant Chemotherapy and Radiotherapy with SBRT Boost for Unresectable Stage III Non-Small Cell Lung Cancer: A Phase I Study. J Thorac Oncol 2017;12:1687-95. [Crossref] [PubMed]
- Peulen H, Franssen G, Belderbos J, et al. SBRT combined with concurrent chemoradiation in stage III NSCLC: Feasibility study of the phase I Hybrid trial. Radiother Oncol 2019; [Epub ahead of print]. [PubMed]
- Timmerman RD, Paulus R, Pass HI, et al. Stereotactic Body Radiation Therapy for Operable Early-Stage Lung Cancer: Findings From the NRG Oncology RTOG 0618 Trial. JAMA Oncol 2018;4:1263-6. [Crossref] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE). Accessed on June 14, 2010. Available online: http://www.eortc.be/services/doc/ctc/ctcae_4.03_2010-06-14_quickreference_5x7.pdf
- Nguyen NP, Bishop M, Borok TJ, et al. Pattern of failure following chemoradiation for locally advanced non-small cell lung cancer: potential role for stereotactic body radiotherapy. Anticancer Res 2010;30:953-61. [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290-6. [Crossref] [PubMed]
- Nagata Y, Hiraoka M, Shibata T, et al. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys 2015;93:989-96. [Crossref] [PubMed]
- Widder J, Postmus D, Ubbels JF, et al. Survival and quality of life after stereotactic or 3D-conformal radiotherapy for inoperable early-stage lung cancer. Int J Radiat Oncol Biol Phys 2011;81:e291-7. [Crossref] [PubMed]
- Bradley JD, El Naqa I, Drzymala RE, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung cancer: the pattern of failure is distant. Int J Radiat Oncol Biol Phys 2010;77:1146-50. [Crossref] [PubMed]
- Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009;75:677-82. [Crossref] [PubMed]
- Ball D, Mai GT, Vinod S, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol 2019;20:494-503. [Crossref] [PubMed]
- Dabaja B, Salehpour MR, Rosen I, et al. Intensity-modulated radiation therapy (IMRT) of cancers of the head and neck: comparison of split-field and whole-field techniques. Int J Radiat Oncol Biol Phys 2005;63:1000-5. [Crossref] [PubMed]
Cite this article as: Huang LW, Lin HY, Lee MS, Chiou WY, Chen LC, Hung SK. Combined stereotactic body radiotherapy with conventionally fractionated radiotherapy successfully managing a locoregionally advanced lung cancer patient: a case report. Ther Radiol Oncol 2020;4:3.