
Overt tumor regression after salvage boron neutron capture therapy (BNCT) for a recurrent glioblastoma patient
Introduction
According to the cancer registration statistics of the Taiwan’s Health Promotion Administration, approximately 700 adults are diagnosed with malignant brain tumors in Taiwan every year. Among them, malignant gliomas accounted for 70% of the cases (1). Glioblastoma, which is classified as a grade IV glioma by the World Health Organization (WHO), is an extremely intractable disease. It has a dismal prognosis, and even after undergoing radical surgery and receiving adjuvant radiation therapy and oral chemotherapy (temozolomide), there is still a high chance of recurrence. In addition, if the treatment is not effective in avoiding tumor recurrence, the survival time is approximately around one year (2). Thus, current treatment strategies for this highly malignant cancer have reached a bottleneck that needs to be overcome urgently.
In the 1950s, Dr. William Sweet, a neurosurgeon in Massachusetts General Hospital in the United States, led the first human clinical trial of boron neutron capture therapy (BNCT) in the world for malignant brain tumors using a research reactor in Long Island, New York [Brookhaven Medical Research Reactor (BMRR)] and performed the world's first human clinical trial. Due to the lack of preciseness of nuclear technology during that time, and the boron-containing drug used [borax (Na2B4O7.10H2O)] did not have good specificity, the study did not succeed in developing a feasible treatment for malignant brain tumors (3). However, it inspired various countries worldwide to pay highly attention on this therapeutic option. These effects were replicated in many countries including Japan, Finland, Sweden, the Netherlands, and the Czech Republic. Various clinical trials of BNCT for malignant brain tumors were successively proposed, which have demonstrated a gradual improvement in medical strategies against these tumors. Especially in clinical treatment of brain tumors in Japan, many cases have reported better disease control rate after receiving BNCT compared to receiving traditional photon therapy. These patients not only survived for more than two years, but also had excellent quality of life (4-7).
Since 2010, Department of Oncology in Taipei Veterans General Hospital and Nuclear Science & Technology Development Center in National Tsing Hua University have been collaborating to conduct several clinical trials of BNCT in Taiwan. In this report, we describe a case with significant response by applying emergent BNCT treatment to a glioblastoma patient. We aim to refine this treatment technology through the management of similar cases in the future (Figure 1).
Case presentation
The patient was a 52-year-old woman with no known history of any underline disease. According to her description, she began to discover that her spatial perception worsened in July, 2015. She fell frequently, her memory deteriorated, and she gradually became unable to use her mobile phone. Since the patient was in Shanghai at the time, she underwent brain computed tomography at the local hospital. The imaging results showed a tumor, approximately 35 mm in size, in the left parietal lobe. Therefore, she was transferred back to Taiwan for subsequent evaluation and treatment. Brain magnetic resonance imaging (MRI) was performed, and revealed a 3.5 cm × 2.7 cm × 3.2 cm cystic lesion located in the left parietal lobe. It was suspected a primary malignant glioblastoma or malignant metastatic lesion. After evaluation and related arrangements, the patient underwent craniotomy on October 11th, 2017 and near total removal of the tumor was achieved. Postoperative pathology reports indicated a highly malignant glioblastoma (WHO Classification IV). Microscopic findings revealed the tumor tested negative for IDH1 mutation, and positive for O6-methylguanine methyltransferase (MGMT) and 35% of MIB-1 index. The patient then received standard adjuvant radiotherapy (6,000 cGy in 30 fractions) combined with chemotherapy with concurrent oral temozolomide (120 mg daily) for a total of six weeks (October 30, 2017–December 8, 2017); this was followed by intensive oral temozolomide for 12 months (300 mg daily for 5 days as a course, and a course for each month). However, in the fourth month of adjuvant treatment, a follow-up MRI showed tumor recurrence around the previous surgical bed, and the patient began to experience right limbs hemiplegia rapidly. Due to the extremely limited selection of other relevant active treatments in this urgent status, a referral for salvage BNCT was recommended.
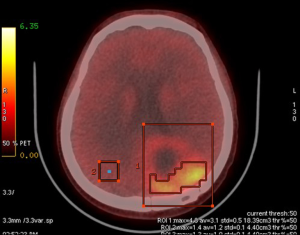
The patient underwent 4-borono-2-18F-fluoro-phenylalanine (FBPA) positron emission tomography (PET) in the Department of Nuclear Medicine at our hospital before BNCT was implemented. The main purpose of this imaging is to use nuclear medicine technology to determine the distribution of boron-containing drug L-(4-10borophenyl) alanine (L-BPA) using radioisotope fluorine-18 to synthesize 4-borono-2-18F-fluoro-phenylalanine (FBPA). Subsequently, PET scanning is used to determine whether the boron-containing drug has been delivered to the tumor site. Through this advanced drug labeling technology, we expect that the activity and concentration of the drug at the tumor site is 2.5-fold or greater than that in normal tissues. On February 3rd, 2017, the patient was evaluated in the Department of Nuclear Medicine at our hospital using this imaging technique. Fortunately, the tumor/normal tissue uptake ratio (T/N ratio) was 2.6. It indicated enough intake of boron-containing drugs by the tumor to an acceptable amount for planning and implementation of BNCT (Figure 2). Since BNCT is not a standard cancer treatment option in Taiwan, we should make an emergent application as for a compassionate treatment to the department of our government in charge. It was approved by the Institutional Review Board (IRB) of Taipei Veterans General Hospital and Taiwan’s Food and Drug Administration (TFDA) thereafter. BNCT was verified legally to be performed in Tsing-Hua University.
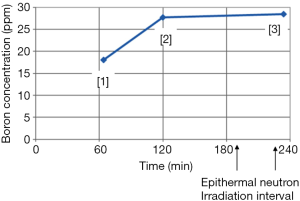
Two weeks before BNCT commenced, computed tomography simulation was done for the design of treatment planning. The patient was scheduled to undergo BNCT on May 11th. Prior to BNCT, we were concerned about the long interval to the approved treatment course, thus, the patient received two-course chemotherapy (intravenous cyclophosphamide 800 mg and vinblastine 9 mg) for tentative tumor control. On the day of treatment, the patient departed Taipei Veterans General Hospital in an ambulance. The patient was in a stable physical condition (Karnofsky performance status score =70). The patient arrived at Tsing-Hua Open Pool Reactor in Hsinchu at 8:30 am on the same day. After undergoing initial position simulation and determining the position of irradiation, the patient received a continuous infusion of L-BPA at 10:00 am. The total dose infused was 450 mg/kg body weight [adjusted from the dose of 500 mg/kg, originally formulated at Kyoto University, Japan (8)]. The infusion formulation was designed for full infusion during the first two hours followed by half infusion rate during the neutron irradiation process, with the expectation of maintaining a constant concentration of boron in the blood (180 to 180 to 90 mg/kg body weight). Since the tumor dose is related to boron concentration in the tumor, which in turn, it is related to boron concentration in the blood. The drug was manufactured by Taiwan Biotech Co., Ltd. and met the Good Manufacturing Practice (GMP) standards; it had also been approved by the Taiwan Ministry of Health and Welfare. After the first and second hour of full dose drug infusion, blood boron concentration was evaluated by using inductively coupled plasma with atomic emission spectroscopy (ICP-AES), which showed values of 18.04 and 27.72 ppm, respectively. In agreement with relevant international studies, the blood boron concentration prior to irradiation was ideally greater than 25 ppm (Figure 3). After two hours of full dosage L-BPA infusion, the dosage of L-BPA was reduced to half of the initial dosage and was delivered for another one hour. Patient then entered the irradiation room and received epithermal neutron irradiation with two portals for approximately 27.5 minutes (under 1.2 Mega-Watt of reactor’s operation). After irradiation, the patient was removed from the irradiation room, and at this last blood collection point, the blood boron concentration was 28.46 ppm (Figure 3). The highest, lowest and average doses received by the tumor (gross tumor volume) were 33.11, 6.71, and 20.44 Gy-E, respectively. In addition, the average dose received by the normal brain tissue was below 2 Gy-E. The patient was observed after the irradiation treatment and monitored for activation of related materials. After relevant tests were completed, she returned to Taipei Veterans General Hospital for follow-up care.
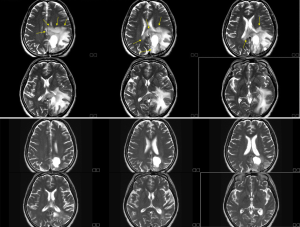
Two weeks after BNCT, we followed brain MRI, and it revealed overt tumor regression with less perifocal edema (Figure 4). Patient felt easy without significant complaint about her physical condition. Previous right-side limb weakness improved significantly also. Secondary followed brain MRI in August still demonstrated stable condition without disease relapse. The patient continues to undergo Bevacizumab to prevent post-BNCT radiation damage for every two weeks. Brain MRI will be followed regularly every three months.
Discussion
The treatment of malignant intracranial glioblastomas has always been a very difficult subject in the field of neuro-oncology. Traditional studies have confirmed that the radicality of surgery has a close prognostic relationship with the age and physical condition of the patient. In recent years, new oral chemotherapy drugs (temozolomide), chemotherapy wafers that are implanted in the tumor site during surgery (Gliadel Wafer), targeted angiogenesis inhibitors (bevacizumab), and other drugs have been actively involved in clinical brain tumor care. However, the efficacy of these new therapeutic drugs was very limited (8-10). Therefore, the development of effective treatment methods to improve disease control is urgently needed in the field of neuro-oncology.
BNCT is a surprisingly attractive targeted treatment that increases brain tumor control and reduces damage to the surrounding normal tissues. There have been many improvements in BNCT and it has produced many preliminary clinical results over the past few decades. In the past, this technology had reached a threshold because of the need of collaboration across multiple disciplines, including clinical oncology, radiation biology, development of boron-containing drugs, nuclear engineering technology, and medical physics. However, this treatment technique has been actively developed in Taiwan in recent years. This is because Taiwan possesses multidisciplinary researchers in various fields to provide professional academic theories, including theories for increasing the concentration of boron-containing drugs in tumors (11), which would improve the effectiveness of the treatment.
In this case, the patient was referred to our department to evaluate the option of BNCT as she was declared to be unresponsive to other treatments. With respect to the rapid tumor recurrence in the patient, she still achieved our standard BNCT assessment, including the special PET system, FBPA-PET, which showed a high uptake capacity of the tumor for boron-containing drugs. This result had two points of significance: (I) the tumor would have an excellent response to BNCT; (II) the tumor had a very high degree of malignancy and showed rapid proliferative status and was in a state of unfavorable disease development. FBPA-PET combines different medical and radiological technologies (12-14). It is not only different from traditional 18F-fluorodeoxyglucose (FDG)-PET, but it also has a special role in the diagnosis of brain tumors because the structure of L-BPA is similar to that of phenylalanine, an essential amino acid in humans. The mode of uptake of amino acids by tumor cells is different from that of glucose. In addition, foreign studies have also reported that the FBPA-PET evaluation technique can provide excellent discriminating ability between necrosis caused by radiotherapy and recurrent brain tumors. In other words, excellent treatment results can be achieved for the patient with the assistance of these professional objective conditions.
After the patient successfully completed BNCT, MRI performed two weeks later found that the tumor tissue that had up taken boron-containing drugs had disappeared almost completely, while normal tissue that did not absorb boron-containing drugs were nearly unaffected. This was different from the traditional X-ray radiotherapy, in which, after irradiation, edema and necrosis develop in the normal tissue. Moreover, BNCT has been shown to have biological advantages over traditional photon therapies in previous studies (15). In addition, BNCT requires less dosage and treatment interval, which is beneficial for patients in poor physical condition. Although BNCT has advantages over conventional photon therapy for brain tumors, some breakthroughs are still needed, including the dose calculation system which differs significantly from that used in conventional photon therapy. Therefore, the biological equivalent doses used in the two systems are not comparable and interchangeable. This is because they have their own independent evaluation systems, and all kinds of related radiation physical dose assessments and measurement techniques must be modified. In addition, at the end of conventional photon treatment, there is often no space for re-irradiating the same place in a short period of time. However, the re-treatment evaluation of BNCT is based on the tolerance of normal tissues, and if this tolerance is not exceeded, BNCT can be repeated. However, dissimilar to conventional X-ray radiotherapy, evaluation of the dose tolerated by normal tissue in BNCT is different from that in conventional constraints of X-ray therapy, which has an independent evaluation system, and the two are not interchangeable (16). For deeper and less heterogeneous tumor lesions, BNCT can employ a multi-angle neutron irradiation treatment mode, which can not only yield a better tumor dose coverage, but can also reduce unnecessary dosing of normal tissue (17).
With respect to targeted treatments (bevacizumab, Avastin®), although the international literature indicates that there is no significant improvement in survival rates for malignant glioblastoma (9), there are considerable researches showing that it can prevent tumor necrosis or pseudoprogression, which are common conditions that appear after BNCT for malignant brain tumors. Therefore, the combination of BNCT and bevacizumab has gradually become a recommended combination therapy (18,19).
With respect to the neutron source for BNCT, there is currently an international aim of developing neutron accelerators to replace atomic reactors. Compared to an atomic reactor, an accelerator can be better controlled, safer, reduce the problems of nuclear waste disposal, and is more appropriate as a formal medical device in a medical institution. Internationally, Japan, the United States, Italy, Israel, and Russia currently have plans of developing accelerator systems for BNCT. In neighboring Japan, accelerator-based BNCT has reached the milestone of completing clinical trials (malignant brain tumors and recurrent head and neck cancers). These are about to become one of the standard choices in oncology near in time (20).
Finally, the prognosis and survival of malignant intracranial glioblastoma patients has always been an important concern in the field of clinical neuro-oncology. In recent years, due to the continuous advancement in molecular diagnostic techniques for malignant glioblastoma, many different subtypes of glioblastoma have been classified; researchers have found that the prognosis of different subtypes has a great variety (2). Thus, determining whether the effect of BNCT on different subtypes is related is an important issue in prognosis research.
In conclusion, we gained important and valuable experience from this case. We believe that BNCT has superior efficacy, stability, safety, and has a less damaging effect on quality of life compared to oral chemotherapy and targeted drugs. Patients should be actively evaluated for the possibility of using BNCT in the early stages of recurrent brain tumors to achieve better disease control. Due to this cancer treatment technology was well developed in Taiwan, it is expected that BNCT will continue to develop through accumulation of experience, and refinement of therapeutic technology. Therefore, BNCT can be widely performed to treat more patients with malignant tumors in the near future.
Acknowledgments
The research group would like to express the gratitude to the following researchers who provided assistance for the study: Professor Shiang-Huei Jiang, Yen-Wen Liu Hsieh and Dr. Hong-Ming Liu from Institute of Nuclear Engineering and Science, Tsing-Hua University; Taiwan Biotech Company for manufacturing of L-BPA; Chi-Wei Chang, Nuclear Pharmacist of Department of Nuclear Medicine, Taipei Veterans General Hospital; Study Nurse: Pei-Yu Lai, and research assistant: Meng-Hsuan Lin; Research Center for Boron Neutron Capture Therapy at Tamkang University.
Funding: Research grant support from Ministry of Science and Technology, Taiwan: MOST 107-2314-B-075-039.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hiroaki Kumada and Yi-Wei Chen) for the series “Boron Neutron Capture Therapy” published in Therapeutic Radiology and Oncology. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2018.10.09). The series “Boron Neutron Capture Therapy” was commissioned by the editorial office without any funding or sponsorship. YML serve as an unpaid editorial board members of Therapeutic Radiology and Oncology from Oct 2017 to Sep 2019. YWC served as the unpaid Guest Editor of the series and serves as an Associate Editors-in-Chief of Therapeutic Radiology and Oncology.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- 2015 Annual Cancer Registration Report in Taiwan, Health Promotion Administration, Ministry of Health and Welfare, Republic of China in Taiwan (December 28th 2017). Available online: https://www.hpa.gov.tw/Pages/List.aspx?nodeid=269
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803-20. [Crossref] [PubMed]
- Sweet WH. The uses of nuclear disintegration in the diagnosis and treatment of brain tumor. N Engl J Med 1951;245:875-8. [Crossref] [PubMed]
- Barth RF, Vicente MG, Harling OK, et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat Oncol 2012;7:146. [Crossref] [PubMed]
- Sauerwein W, Wittig A, Moss R, et al. Neutron Capture Therapy. Springer 2012;41-54.
- Kankaanranta L, Seppälä T, Koivunoro H, et al. L-boronophenylalanine-mediated boron neutron capture therapy for malignant glioma progressing after external beam radiation therapy: a Phase I study. Int J Radiat Oncol Biol Phys 2011;80:369-76. [Crossref] [PubMed]
- Kawabata S, Miyatake S, Nonoguchi N, et al. Survival benefit from boron neutron capture therapy for the newly diagnosed glioblastoma patients. Appl Radiat Isot 2009;67:S15-8. [Crossref] [PubMed]
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96. [Crossref] [PubMed]
- Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 2014;370:709-22. [Crossref] [PubMed]
- Ashby LS, Smith KA, Stea B. Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: a systematic literature review. World J Surg Oncol 2016;14:225. [Crossref] [PubMed]
- Yang FY, Chen YW, Chou FI, et al. Boron neutron capture therapy for glioblastoma multiforme: enhanced drug delivery and antitumor effect following blood-brain barrier disruption induced by focused ultrasound. Future Oncol 2012;8:1361-9. [Crossref] [PubMed]
- Wang HE, Liao AH, Deng WP, et al. Evaluation of 4-borono-2-18F-fluoro-L-phenylalanine-fructose as a probe for boron neutron capture therapy in a glioma-bearing rat model. J Nucl Med 2004;45:302-8. [PubMed]
- Hanaoka K, Watabe T, Naka S, et al. FBPA PET in boron neutron capture therapy for cancer: prediction of (10)B concentration in the tumor and normal tissue in a rat xenograft model. EJNMMI Res 2014;4:70. [Crossref] [PubMed]
- Watabe T, Hanaoka K, Naka S, et al. Practical calculation method to estimate the absolute boron concentration in tissues using 18F-FBPA PET. Ann Nucl Med 2017;31:481-5. [Crossref] [PubMed]
- Coderre JA, Makar MS, Micca PL, et al. Derivations of relative biological effectiveness for the high-let radiations produced during boron neutron capture irradiations of the 9L rat gliosarcoma in vitro and in vivo. Int J Radiat Oncol Biol Phys. 1993;27:1121-9. [Crossref] [PubMed]
- Hsu SM, Hung CH, Liao YJ, et al. Feasibility Study on Applying Radiophotoluminescent Glass Dosimeters for CyberKnife SRS Dose Verification. PLoS One 2017;12:e0169252 [Crossref] [PubMed]
- Lee JC, Chuang KS, Chen YW, et al. Preliminary dosimetric study on feasibility of multi-beam boron neutron capture therapy in patients with diffuse intrinsic pontine glioma without craniotomy. PLoS One 2017;12:e0180461 [Crossref] [PubMed]
- Furuse M, Kawabata S, Kuroiwa T, et al. Repeated treatments with bevacizumab for recurrent radiation necrosis in patients with malignant brain tumors: a report of 2 cases. J Neurooncol 2011;102:471-5. [Crossref] [PubMed]
- Miyatake S, Kawabata S, Hiramatsu R, et al. Boron neutron capture therapy with bevacizumab may prolong the survival of recurrent malignant glioma patients: four cases. Radiat Oncol 2014;9:6. [Crossref] [PubMed]
- Barth RF, Zhang Z, Liu T. A realistic appraisal of boron neutron capture therapy as a cancer treatment modality. Cancer Commun (Lond) 2018;38:36. [Crossref] [PubMed]
Cite this article as: Lan TL, Chou FI, Huang WS, Lin KH, Lee YY, Pan PS, Kuo YC, Hsu SM, Chang FC, Liang ML, Lee JC, Lin SC, Liu YM, Chao Y, Chen YW. Overt tumor regression after salvage boron neutron capture therapy (BNCT) for a recurrent glioblastoma patient. Ther Radiol Oncol 2018;2:48.


