
Adjuvant radiotherapy using intensity-modulated radiotherapy (IMRT) technique for localized extraskeletal osteosarcoma (ESOS): case report and literature review
Introduction
Extraskeletal osteosarcoma (ESOS) is a malignant mesenchymal neoplasm that produces osteoid bone, or chondroid material without demonstrable attachments to bone or periosteum (1). These tumors are distinct from conventional osteosarcoma. The diagnosis of ESOS accounts for 2–5% of osteosarcomas and 1% of all soft-tissue sarcomas (2). The etiology is not fully established, although some factors of preceding trauma or previous radiation exposure history were postulated to be risk factors (3). Surgical resection is considered the mainstay curative treatment. However, the relapse rate is over 75% (4). Due to its rarity and poor prognosis, there is a lack of universal consensus on optimal treatment strategy.
In this article, we demonstrated that a patient with ESOS receiving surgical resection followed by adjuvant radiotherapy using intensity-modulated radiotherapy (IMRT) technique. We also reviewed the literatures regarding the treatment options, clinical outcomes of ESOS, and the role of adjuvant radiotherapy.
Brief history
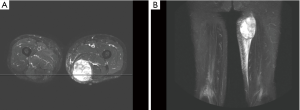
This 70-year-old gentleman has the underlying disease of hypertension and a medical history of cerebrovascular accident (CVA) without neurologic sequela. He presented with a swelling mass at his left thigh and initially sought for medical advice at an outside facility. Cellulitis-like change was noted and resolved, but the mass enlarged rapidly in 3 months. He was referred to our hospital for further management. The patient denied previous history of major trauma, dysfunction, or any radiation exposure to his left thigh. On physical examination, a non-tender tumor up to 10 cm was noted at the medial aspect of left thigh. The patient provided that he just received magnetic resonance imaging (MRI) at outside medical facility. The images revealed a hypervascular tumor at posterior medial aspect as left thigh, with invasion to semimembranosus and adductor magnus muscle (Figure 1). Perilesional soft tissue edema was noted but there was no definitive bone lesion nor lymphadenopathy. After explanation and discussion, mesenchymal origin tumor was highly suspected. The patient then underwent tumor excision on October 25, 2016. Intra-operatively, a hypervascular tumor was identified and specimen measuring 10.5 cm × 8.0 cm × 5.3 cm was submitted to pathology review. Grossly, the tumor was multicystic with hemorrhage. Microscopic finding showed pleomorphic tumor cells with occasional bizarre giant tumor cells and frequent mitoses. Foci of chondroid formation and occasional malignant osteoid were also found. Immunohistochemical study demonstrated the negative result for cytokeratin, MDM2, CDK4, SMA, nor desmin. S100 was only scantly positive. Chest CT scan showed no definite lung lesion. Taken together the results of histological pattern and immunohistochemical staining, the final diagnosis was extra-skeletal osteosarcoma, T2bN0M0, FNCLCC grade III. The section margin was not involved by the tumor, but the closest distance to tumor was 1.2 mm. At multidisciplinary conference, the patient was suggested to receive adjuvant radiotherapy (RT) for decreasing the risk of local recurrence, but adjuvant chemotherapy was not considered for this patient.
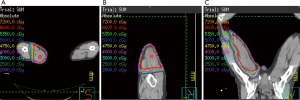
After wound healing, the stitches were removed 2 weeks after tumor excision, and post-operative RT was arranged 1 month after operation. The patient was immobilized using a vacuum bag. The RT treatment consisted of 2 stages. The prescribed dose of the first stage was 50 gray (Gy) in 25 fractions. The initial clinical target volume (CTV) included the tumor bed, operation scar, and surgically disturbed tissues, with a 4.5 cm margin in the longitudinal axis and a 1.5 cm margin in the transverse plane. The second stage was sequential boost for tumor bed. The prescribed dose was 16 Gy in 8 fractions to a cumulative dose of 66 Gy. The boost CTV included the tumor bed and a 1.5 cm margin in all directions. The planning target volume (PTV) was CTV with 0.5 cm margin in all directions. We used IMRT technique to treat the patient with good conformity. Photon energy was 6 megavoltage. Beam configurations consists of 4 beam portals. Maximal dose was 109.2% of prescribed dose and the PTV coverage with 95% of the prescribed dose was achieved. The isodose lines were shown in Figure 2.
Toxicities resulting from radiation were assessed using the National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE) scale version 4.0 (5). The patient had acute toxicities of grade 2 dermatitis and grade 1 edema during RT. The symptom resolved after topical ointment. Follow-up MRI was performed 3 months after RT. The result showed hyperemic post-operative change without residual/recurrent tumor. The patient reported grade 1 fibrosis, and minimal joint stiffness after recovery. One episode of cellulitis was noted in April, 2017. The infection resolved after adequate anti-microbial treatment. The pus discharge was negative for malignant cell. Further follow-up images study of CT scan was arranged. The patient was free from recurrence or metastasis of sarcoma for 2 years after RT.
Discussion
First reported in 1941 by Wilson (6), ESOS is a rare mesenchymal tumor arising in soft tissue. It accounts for 1% of all soft tissue sarcomas and nearly 2–5% of all osteosarcomas (6,7). Pathological appearance and grades are well established and are based on cell morphology and mitotic rates. To be classified as ESOS, it must arise in the soft tissue (not attached to bone or periosteum), have a uniform sarcomatous pattern, and produce osteoid and/or cartilage matrix (8,9). The etiology of ESOS is not fully understood. Some series of study reported an association with preceding trauma in 12–13% cases and prior history of radiotherapy in 5–10% cases (7,10). However, the patient in presentation had no previous history of trauma or radiation exposure.
Compared with primary osteosarcoma of bone, the epidemiology and prevalence of ESOS are different. Primary osteosarcoma of bone occurs in much younger patients, with a peak incidence in the second decade of life (11,12). On the contrary, ESOS is reportedly uncommon under the age of thirty. Median age was typically in the sixth decade of life. Additionally, the male predominance seen in primary osteosarcoma is not found among patients with ESOS (12). It is estimated that primary osteosarcoma of bone is the most common malignant bone tumor, which is contrast to the rarity of ESOS among soft-tissue sarcomas (12).
The outcome of ESOS was poor after surgical resection. Historical studies showed overall survival at 5 years ranged from 25% to 77% (2,3). ESOS also has high rates of local recurrence and distant metastases. The reported rates were 69% for local recurrence and 80% for distant metastases (2,13). Adjuvant RT is considered to decrease local recurrence in soft-tissue sarcomas (14). However, most studies regarding radiation and soft-tissue sarcoma do not include cases of ESOS. Sordillo et al. (15) retrospectively analyzed the outcome of ESOS, and recurrence rate was lower in patients who underwent wide excision followed by adjuvant RT than wide excision alone. The median time to recurrence was 7 months in patients who received operation alone compared to 12 months in patients receiving operation followed by RT (15). However, the dose, fractionation, technique and adverse effects are not clearly reported. Longhi et al. (7) retrospectively reviewed 266 patients with ESOS. RT was administered about one third in patients with ESOS. In analysis, there was no difference in the choice of RT administration according to age, size of primary tumor. Notably, RT seems to give an advantage in those patients with tumor >5 cm and R0 margins, whereas no benefit was seen in patients with R1 margins. The results indicated that inadequate surgery many not be overcome by RT (7).
Although the use of RT is also preferred in patients with deep, high-grade, large tumors regardless of the ability to achieve adequate margins, previous studies raised an issue of poor wound healing when radiation was given in combination with surgery (16,17). Some old studies demonstrated complication rates ranging from 22% to 35% (16,17). Werier et al. (18) developed a reproducible radiation healing-impaired deep wound animal model. Impaired healing was still evident until 6 weeks after intervention. Clinically, the impact of time interval (TI) between surgery and start of adjuvant RT was assessed from French Sarcoma Group (19). Data from 1,131 patients were retrospectively reviewed and the median TI was 82 days (range, 18–346). With a TI of 19–39, 40–79, 80–119, and ≥120 days, 10-year overall survivals were 72.8%, 60.7%, 66.4%, and 62.1% (P=0.347), and 10-year local relapsed-free survivals were 65.3%, 55.5%, 56.9%, and 61.2% (P=0.465), respectively. The author concluded that the TI between surgery and start of adjuvant RT did not seem to affect the outcomes. However, RT should be administrated as early as possible if healing is already obtained, especially for patients with high risk of local recurrence (19). LeBrun et al. (20) analyzed the predictors of wound complications following radiation and surgical resection of soft tissue sarcoma. Sixty-five patients representing 67 cases of soft tissue sarcoma were identified. The rates of major wound complications and any wound complications were 21% and 33%, respectively. After adjusting for radiation timing, the result revealed that diabetes, grade 2 or above radiation dermatitis, and the use of IMRT were associated with an decreased risk of any wound complication on multivariable analysis (20). These data suggest that radiation modality and radiation dermatitis also play the roles of predictors wound complications in patients with sarcoma. In this case, the patient received adjuvant RT after the surgical wound was healed. Surgical scar was marked at simulation. Radiation was administrated via IMRT technique with skin flashing in treatment fields to achieve good conformity and minimize the possible toxicity.
The role of chemotherapy for ESOS was not fully established. Some institutes reported using cisplatin as a regimen but the response was not satisfied (12,15). Lee et al. (21) reported the outcome of 40 patients with ESOS. The 5-year survival was 37%, and the use of adjuvant chemotherapy did not affect survival. Ahmad et al. (22) reported that cisplatin-based chemotherapy is not active against ESOS and the response to doxorubicin is also poor. The 5-year disease-specific survival rate was 46%. Response rate to chemotherapy was only 19%. They asserted that ESOS should be viewed as therapeutically distinct from conventional osseous osteosarcoma. In recent study, Goldstein-Jackson et al. (12) suggested that ESOS is better treated with more aggressive multi-agent chemotherapy. The study on 17 patients from the Cooperative Osteosarcoma Study Group (COSS) reported a favorable 3-year OS of 77% after multimodal treatment and chemotherapy, including doxorubicin, ifosfamide, cisplatin, and methotrexate (12). The role of immunotherapy is also under investigation. Maki et al. (23) treated patients with synovial sarcoma with ipilimumab (anti-CTLA4) in a pilot, phase II study. Unfortunately, the trial was closed due to poor patient accrual. Among the 6 patients enrolled, no serologic evidence of an immune response was seen and all patients demonstrated disease progression after therapy (23,24). To our knowledge, there is still no direct clinical trial to assess the response of immunotherapy to the specific disease entity of ESOS. However, one case report from Matsuo et al. (25) showed the unique histological features of partial spontaneous regression of malignancy of the primary lesion of ESOS. In pathological findings, tumor cells decreased gradually from the central area and were replaced by fibrocollagenous tissue with no sarcoma cells. CD8+, T-cell-restricted intracellular antigen-1 (TIA-1)+, granzyme B+ T lymphocytes appeared to infiltrate the mass lesion. The author hypothesized that the immunological system was likely to be involved via T lymphocytes in triggering spontaneous regression (25). Further understanding of this phenomenon and the mechanism of lymphocyte infiltration may provide the possible immunological treatment strategy to ESOS. In our case, pleomorphic tumor cells were seen in multicystic tumors and spontaneous regression in the central part of tumor was not identified. The use of combination regimen and immunological therapy remains a subject for further exploration.
Conclusions
In summary, ESOS represents an unusual soft-tissue sarcoma that occurs in the elderly. Pathologic diagnosis is confirmed by the presence of neoplastic osteoid, uniform sarcomatous pattern, without attachment to bone or periosteum. Limb-sparing resection is mainstay treatment but the prognosis is poor. Our case report indicated that adjuvant radiotherapy with sequential boost to 66 Gy via modern IMRT technique is a safe and effective treatment option. The high radiation dose in combination with surgery should be considered to achieve lower recurrence rate without major adverse effects jeopardizing limb function.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2018.10.02). SHK serves as an Associate Editors-in-Chief of Therapeutic Radiology and Oncology from Oct 2017 to Sep 2019. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fine G, Stout AP. Osteogenic sarcoma of the extraskeletal soft tissues. Cancer 1956;9:1027-43. [Crossref] [PubMed]
- Bane BL, Evans HL, Ro JY, et al. Extraskeletal osteosarcoma. A clinicopathologic review of 26 cases. Cancer 1990;65:2762-70. [Crossref] [PubMed]
- Allan CJ, Soule EH. Osteogenic sarcoma of the somatic soft tissues. Clinicopathologic study of 26 cases and review of literature. Cancer 1971;27:1121-33. [Crossref] [PubMed]
- Huvos AG. Osteogenic sarcoma of bones and soft tissues in older persons. A clinicopathologic analysis of 117 patients older than 60 years. Cancer 1986;57:1442-9. [Crossref] [PubMed]
- National Cancer Institute, US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) version 4.0, 2009.
- Wilson H. Extraskeletal Ossifying Tumors. Ann Surg 1941;113:95-112. [Crossref] [PubMed]
- Longhi A, Bielack SS, Grimer R, et al. Extraskeletal osteosarcoma: A European Musculoskeletal Oncology Society study on 266 patients. Eur J Cancer 2017;74:9-16. [Crossref] [PubMed]
- McCarter MD, Lewis JJ, Antonescu CR, et al. Extraskeletal osteosarcoma: analysis of outcome of a rare neoplasm. Sarcoma 2000;4:119-23. [Crossref] [PubMed]
- Roller LA, Chebib I, Bredella MA, et al. Clinical, radiological, and pathological features of extraskeletal osteosarcoma. Skeletal Radiol 2018;47:1213-20. [Crossref] [PubMed]
- Chung EB, Enzinger FM. Extraskeletal osteosarcoma. Cancer 1987;60:1132-42. [Crossref] [PubMed]
- Nystrom LM, Reimer NB, Reith JD, et al. The Treatment and Outcomes of Extraskeletal Osteosarcoma: Institutional Experience and Review of The Literature. Iowa Orthop J 2016;36:98-103. [PubMed]
- Goldstein-Jackson SY, Gosheger G, Delling G, et al. Extraskeletal osteosarcoma has a favourable prognosis when treated like conventional osteosarcoma. J Cancer Res Clin Oncol 2005;131:520-6. [Crossref] [PubMed]
- Thampi S, Matthay KK, Boscardin WJ, et al. Clinical Features and Outcomes Differ between Skeletal and Extraskeletal Osteosarcoma. Sarcoma 2014;2014:902620 [Crossref] [PubMed]
- Beane JD, Yang JC, White D, et al. Efficacy of adjuvant radiation therapy in the treatment of soft tissue sarcoma of the extremity: 20-year follow-up of a randomized prospective trial. Ann Surg Oncol 2014;21:2484-9. [Crossref] [PubMed]
- Sordillo PP, Hajdu SI, Magill GB, et al. Extraosseous osteogenic sarcoma. A review of 48 patients. Cancer 1983;51:727-34. [Crossref] [PubMed]
- Cannon CP, Ballo MT, Zagars GK, et al. Complications of combined modality treatment of primary lower extremity soft-tissue sarcomas. Cancer 2006;107:2455-61. [Crossref] [PubMed]
- Tseng JF, Ballo MT, Langstein HN, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol 2006;13:1209-15. [Crossref] [PubMed]
- Werier J, Ferguson P, Bell R, et al. Model of radiation-impaired healing of a deep excisional wound. Wound Repair Regen 2006;14:498-505. [Crossref] [PubMed]
- Fourquet J, Sunyach MP, Vilotte F, et al. Time interval between surgery and start of adjuvant radiotherapy in patients with soft tissue sarcoma: A retrospective analysis of 1131 cases from the French Sarcoma Group. Radiother Oncol 2016;120:156-62. [Crossref] [PubMed]
- LeBrun DG, Guttmann DM, Shabason JE, et al. Predictors of Wound Complications following Radiation and Surgical Resection of Soft Tissue Sarcomas. Sarcoma 2017;2017:5465130 [Crossref] [PubMed]
- Lee JS, Fetsch JF, Wasdhal DA, et al. A review of 40 patients with extraskeletal osteosarcoma. Cancer 1995;76:2253-9. [Crossref] [PubMed]
- Ahmad SA, Patel SR, Ballo MT, et al. Extraosseous osteosarcoma: response to treatment and long-term outcome. J Clin Oncol 2002;20:521-7. [Crossref] [PubMed]
- Maki RG, Jungbluth AA, Gnjatic S, et al. A Pilot Study of Anti-CTLA4 Antibody Ipilimumab in Patients with Synovial Sarcoma. Sarcoma 2013;2013:168145 [Crossref] [PubMed]
- Tseng WW, Somaiah N, Engleman EG. Potential for immunotherapy in soft tissue sarcoma. Hum Vaccin Immunother 2014;10:3117-24. [Crossref] [PubMed]
- Matsuo T, Shimose S, Kubo T, et al. Extraskeletal osteosarcoma with partial spontaneous regression. Anticancer Res 2009;29:5197-201. [PubMed]
Cite this article as: Huang MS, Kuo SH, Wang CC. Adjuvant radiotherapy using intensity-modulated radiotherapy (IMRT) technique for localized extraskeletal osteosarcoma (ESOS): case report and literature review. Ther Radiol Oncol 2018;2:45.


