
A retrospective bilateral breast proton pencil beam scanning and photon volumetric arc therapy planning comparison
Highlight box
Key findings
• Pencil beam scanning (PBS) with protons for bilateral breast cancer patients has better sparing of the heart and lungs compared to volumetric arc therapy (VMAT) for whole breast, chest wall and expander patients. For expanders, this finding holds regardless of whether the PBS plan treats through or around the magnet in the expander.
What is known and what is new?
• PBS with protons provides better sparing of heart and lungs compared to VMAT in a blinded study which is in agreement with the results of other investigators.
• We have extended the analysis to include expanders as well as whole breast and chest wall.
What are the implications, and what should change now?
• Bilateral breast patients, particularly younger ones, should be treated with proton therapy if possible.
Introduction
Breast cancer is the most common cancer in women with approximately 2.3 million women diagnosed annually and 685,000 deaths globally (1). Advanced bilateral breast cancers with local and regional nodal involvement are rare accounting for a few percent (2) of breast cancer patients. These patients are typically treated with mastectomy as well as chemotherapy and radiation. Prognosis is generally favorable but is significantly inferior to unilateral breast cancers (3).
Radiation planning for bilateral breasts poses several challenges. Obtaining good conformality, homogeneity and sparing organs at risk, particularly heart and lung, are of concern. A recent publication summarizes the rationale for protons in breast cancer treatments and reviews much of the literature (4). While advances in X-ray radiation therapy in the past decades from 3D to intensity modulated radiation therapy (IMRT) to volumetric arc therapy (VMAT) and tomotherapy have improved photon distributions, proton therapy has been shown to provide the best distributions. Specifically, Jimenez et al. (5) compared pencil beam scanning (PBS) plans with 3D photon plans for patients who had implants and found PBS plans result in a reduction in lung and heart doses and improved homogeneity. Subsequently, Vyfhuis et al. (6) extended the study for bilateral breast patients and compared PBS and VMAT plans for 3 different cases. They found that PBS offers identical target coverage compared to VMAT plans but with reduced dose to heart and lungs. Sun et al. (7) extended their work to compare IMRT, VMAT, tomotherapy, and PBS plans for 11 patients with bilateral breast cancer. They found that PBS plans reduced mean and low dose volumes to heart, left ventricle and left inferior descending artery.
In this study, we performed a blinded comparison of patients between VMAT and PBS plans. We chose VMAT because it is the standard approach at the photon sites and because the work of Sun et al. (7) demonstrate that photon plans, regardless of delivery modality, provide similar distributions compared to PBS plans.
The use of expanders and their associated high atomic number magnets may perturb the proton distribution and invalidate the comparisons to photon plans. We extend the plan comparisons to include breast expanders in addition to whole breast and implants that were studied by previous investigators. Our planning techniques for the patient with breast expanders are discussed in detail. We present this article in accordance with the STROBE reporting checklist (available at https://tro.amegroups.com/article/view/10.21037/tro-22-23/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of the Western Institution Review Board (IRB) (No. PC 2016-01) and individual consent for this retrospective analysis was waived.
Two independent centers, ProCure Proton Therapy Center in Somerset, NJ, USA and Memorial Sloan Kettering Cancer Center in New York, NY, USA, using different treatment planning systems were involved in this retrospective case-controlled study. Five treated bilateral breast cancer patients were retrospectively selected as representative of a range of patient types (e.g., expander, chest wall only, intact breast) from the proton center from April 2018 to February 2020 under an IRB exempted protocol, the patients’ Digital Imaging and Communications in Medicine (DICOM) files including CT and structure were anonymized and sent to a physicist at the photon site along with the prescription dose. The plan and dose records for the clinical plan were kept at the proton site to ensure that the physicist at the photon site was blinded to the original PBS distribution. Patients included intact breast, post mastectomy patients with chest wall only, and with expanders (Mentor Artoura, Mentor Worldwide, Irvine, CA, USA). All patients had disease that included nodal volumes. All patients were immobilized supine in an alpha cradle with hands over their heads. Patients were scanned in a large bore CT (GE Lightspeed, General Electric Healthcare, Wauwatosa, WI, USA) using a 2.5 mm slice thickness.
All clinical target volumes (CTVs) were generated by the attending physician following the RADCOMP guidelines (8) and were peer reviewed (9) prior to planning. The following volumes were defined. The total CTV was the sum of all CTV volumes including chest wall and nodes. An optimization structure CTV-Skin was defined by a 5 mm crop from the skin surface, excluding rib and intercostal muscle. A planning volume called PTV-Skin was defined as CTV-Skin + 7 mm but 4 mm posteriorly in the region outside lung, excluding rib, intercostal muscle and cropped 5 mm from skin surface since none of the patients had involvement in the dermis. Pulling the volume off the skin was done to reduce skin dose during optimization as proton patients have greater skin toxicity than photon patients (10).
Proton plans were generated using Raystation version 9B (Raysearch, Stockholm, Sweden) using a Monte Carlo dose engine for treatment on an IBA Proteus Plus system (Ion Beam Applications, Louvain-La-Neuve, Belgium) with a universal nozzle and a 7.5 cm range shifter on a 40×30 cm2 snout. All proton plans were generated in Gy (RBE) using a generic RBE (11) of 1.1. Proton plans were a combination of AP beams and matched LAO or RAO en face fields typically at 30 degrees off the vertical and two laterally shifted isocenters. Fields were planned with single field uniform dose (SFUD) except in the volumes where there was overlap between fields. A gap structure, 2 cm wide, ensured a smooth gradient between the match fields. Hence the plans were optimized using a combination of SFUD for most of the volume but using multifield optimization (MFO) in the junction between the two fields. All proton plans were peer reviewed before treatment and were generated with a 3 mm dose grid. While the same physicist generated the comparative photon plans, the original clinical proton plans were generated by different planners based upon the proton center’s planning procedures and criteria. The clinical planning goals were different between VMAT and PBS plans and are summarized in Table 1. The goals are based upon what is clinically achievable.
Table 1
| Location | Organ at risk | |
|---|---|---|
| VMAT | PBS | |
| Each lung | V5 <95% (80–100%); V10 <60%; V20 <25% |
V5 <35%; V20 <16% |
| Both lungs | V5 <95% | V5 <20% |
| Heart | Mean dose <7 Gy | Mean dose <1 Gy |
The planning goals reflect institutional practice based upon what is frequently achievable. PBS, pencil beam scanning; VMAT, volumetric arc therapy.
Patient characteristics are summarized in Table 2 which shows the TNM staging status, age, target description, prescription doses and PTV volumes. For this planning study, only the initial phase of the plan which covered both breasts and involved nodes. The boost volumes were excluded because differences in electron boost versus photon and proton boost could confound the results.
Table 2
| Patient | Cancer stage | Cancer type | Age, years | CTV description | Original Rx (including boost) | PTV-skin volume (cm3) |
|---|---|---|---|---|---|---|
| 1 | T2N1M0 | Invasive ductal carcinoma | 50 | Whole breast + nodes | 50 Gy/25 | 4,866 |
| 2 | T3N1M0 | Invasive lobular carcinoma | 50 | Expanders + nodes | (45+5.4) Gy/28 | 1,941 |
| 3 | T3N2aM0 (right); TN21aM0 (left) | Invasive carcinoma | 75 | Chest wall + nodes | 50 Gy/25 | 4,431 |
| 4 | T1N0M0 | Invasive ductal carcinoma | 67 | Chest wall + nodes | (50+10) Gy/30 | 2,585 |
| 5 | T1cM1N0 | Ductal carcinoma in situ | 51 | Whole breast + nodes | (50+16) Gy/33 | 3,431 |
The prescription includes the boosts if applicable, but only the initial phase was chosen for this comparison study. CTV, clinical target volume; Rx, prescription; PTV, planning target volume.
We were particularly interested in patient #2, who had bilateral breast expanders. Since protons are sensitive to range uncertainties, the addition of a 1.27 cm diameter 0.48 cm thick neodymium high density (7 g/cm3) magnet inhomogeneity in the expander creates an additional complication in treating breast patients with expanders. To our knowledge, there have been no publications about treating bilateral expanders with protons. Hence, we describe details of our planning technique for patients with bilateral breast expanders.
Our initial treatment approach for unilateral breast with expanders was to treat around the magnet following the technique (12-14) which we refer to as the “shoot around” approach. Briefly, three fields are used to treat the target. For each breast, a single AP field treats the superior nodes, separate fields treat most of the lateral and medial aspects of the field using SFUD. However, the magnet and titanium housing have a 5 mm expansion in two dimensions and a 1 cm anterior-posterior expansion. The fields junction with each other using multi-field optimization. To evaluate these plans, we introduced an additional structure called CTV-skin_Eval which consisted of the CTV subtracting the expander with a negative 5 mm margin which can be pictured as the expander with a 5 mm rind extending into the saline solution. The margin was chosen to coincide with the setup margin.
Our new technique, which we designate the “shoot through” technique, used a two-beam approach per breast wherein both beams treated the entire target volume uniformly with the beam transmitting through the magnet to treat the chest wall distal to it. Plans were generated with robustness to setup variations of up to 5 mm and range uncertainty of ±3.5%. For delivery, the magnet was set up to 2 mm allowing a variation in bony anatomy of up to 5 mm. All proton plans were evaluated by a physicist for robustness of CTV coverage to ensure V95 >95%.
The use of Monte Carlo treatment planning enabled us to accurately model treating through the magnet following the method described by Mutter et al. (15). Initially, we obtained a sample expander which was CT scanned and a test plan was generated to verify that the Monte Carlo model matched the measured distribution within a 3.5% range uncertainty using an approach that was originally used for end-to-end testing using animal tissues (16). Briefly, a plan was generated and different thicknesses of water equivalent material were incrementally added until the distribution measured on the ion chamber array matched the planned distribution. A gamma analysis (3%, 3 mm) was used to confirm that the beam transmission through the phantom matched the treatment planning system. Having validated the model for our sample expander, we created models of the expander magnet and needle guard for a range of different expanders based upon each manufacturer’s specification.
One key difference between the “shoot around” and “shoot through” methods was delivery time. The shoot around technique required 3 fields each day whereas the shoot through technique could be delivered with alternating single fields each day which significantly reduces delivery time.
The photon center often uses deep inspiration breath hold (DIBH) for treating bilateral breasts if the patient can perform DIBH reliably (17), but the proton site only uses free breathing plans. For this study, we used the free breathing scans but we expect that the impact of DIBH would not fundamentally change the results of our study. Photon plans were generated using the Eclipse treatment planning system (AAA algorithm, version 15, Varian Medical Systems, Palo Alto, CA, USA) and VMAT using a technique described elsewhere (18). Plans were generated using 6 MV beams and 2.5 mm dose grid on a Truebeam employing multiple partial co-planar arcs. Typically, two planes of arcs were used, to cover the superior and inferior portions of the breast. Smaller arcs were used to carve out different segments of the distribution (e.g., around the arms) to maximize dose homogeneity. All plans were generated with 10 different arcs and a single isocenter.
Plans were generated to the same PTV as used in the proton plans described above. All plans were normalized to the same target coverage to cover the PTV-skin with the prescription isodose level, following the practice at the photon site. The proton site used a slightly more conservative goal of V95 >95% to the PTV-skin clinically but the data were renormalized to meet the coverage goals at the photon site and allow for a fair dose metric comparison.
Plans were compared for conformality and homogeneity (19). The homogeneity index (HI) is defined following the RTOG definition as
where Imax is the maximum isodose and RI is the reference isodose, chosen as 95%, and the conformality index (CI)
where RI is the volume of the reference isodose (95%) and TV is the target volume.
Lung and heart doses were evaluated as well as well as max doses to different organ at risks (OARs). The entire set of metrics is provided in Table 3 for both proton and photon patients.
Table 3
| Dosimetric parameter | VMAT, mean (range) | PBS, mean (range) | P value |
|---|---|---|---|
| CI | 1.04 (0.93–1.17) | 1.06 (0.99–1.15) | 0.51 |
| HI | 1.21 (1.17–1.27) | 1.12 (1.11–1.14) | 0.02 |
| PTV-skin | |||
| D95 (%Rx) | 99.5 (93.8–104.1) | 101.8 (99.8–104.4) | 0.375 |
| V95 (%) | 98.4 (93.3–100) | 99.1 (98.5–99.9) | 0.175 |
| Total lung | |||
| V5 (%) | 96.7 (94.8–99.0) | 39.0 (35.8–42.3) | 0.0000174 |
| V10 (%) | 63.3 (57.8–67.9) | 28.3 (25.7–29.7) | 0.000763 |
| V20 (%) | 27.4 (24.1–31.5) | 13.9 (9.3–15.9) | 0.0004225 |
| V30 (%) | 11.3 (8.9–12.4) | 6.2 (6.2–7.3) | 0.022063 |
| V40 (%) | 2.1 (0.7–3) | 2.3 (0.8–3.2) | 0.0778 |
| Mean dose [Gy (RBE)]/(%Rx) | 15.7 (15.2–16.5)/32.5 (30.4–34.4) | 7.7 (6.3–8.4)/16.6 (15.5–18.6) | 0.000264 |
| Heart | |||
| Mean dose [Gy (RBE)] | 11.4 (10.3–13.3) | 0.69 (0.21–1.15) | 0.000002151 |
| V5 (%) | 98.5 (95.4–100) | 3.5 (1.0–6.7) | 0.00000669 |
| V20 (%) | 17.2 (7.6–39.1) | 0.55 (0.14–0.85) | 0.05337 |
| Thyroid mean dose [Gy (RBE)] | 33.3 (4.78–47.0) | 21.5 (3.5–37.7) | 0.13 |
| Liver mean dose [Gy (RBE)] | 7.9 (6.1–10.7) | 0.53 (0.2–1.0) | 0.000166 |
| Esophagus max dose [Gy (RBE)] | 43.8 (41.0–46.2) | 40.0 (35.9–41.6) | 0.030 |
The mean value along with the range of values for 5 patients are provided. VMAT, volumetric arc therapy; PBS, pencil beam scanning; CI, conformality index; HI, homogeneity index; PTV, planning target volume; Rx, prescription.
Statistical analysis
For each set of dosimetric endpoints, a paired two tail t-test was used to establish the P value between the proton and X-ray arms. Each dosimetric endpoint was taken as a continuous variable and we assumed that the distribution of different dosimetric endpoints was normally distributed.
Results
Dosimetric endpoints
Table 3 summarizes target coverage and OAR dosimetric endpoints along with the corresponding P value. In general, coverage is nearly identical between PBS and VMAT and there are no significant differences between the different target coverage metrics. Conversely, many OARs are dramatically better spared with PBS than VMAT. Specifically, for lung and heart, the volume of lung receiving a particular dose differs significantly for lower doses (e.g., V5, V20) since protons treat a smaller volume at a given isodose level because of their finite range. In contrast at higher doses, e.g., V40, most of the lung is spared in both plans, and there is also no significant difference. The mean liver dose is also reduced with protons as there is no exit dose into the liver. The OARs that are downstream from the target are spared with protons and those that are near the targets are not. For other OARs, the dosimetric endpoints are largely not significantly different, or in the case of spinal cord, within constraints and clinically irrelevant.
Sample distribution comparison
Figure 1 shows the radiation distribution for a PBS and VMAT plan for patient #2. Figure 2 shows the corresponding DVH comparison. The finite range of the protons permit both improved conformality and homogeneity compared to VMAT. Sparing of the heart, liver and lung are significant, particularly at lower isodose levels. Specifically, lung V5, V10, V20 and V30 and mean lung dose are all significantly (P<0.05) less with PBS that VMAT. Similarly, mean heart dose, heart V5 and mean lever dose are also significantly spared (P<0.05).
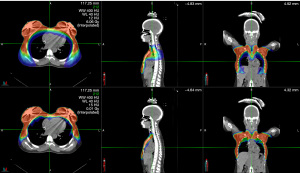
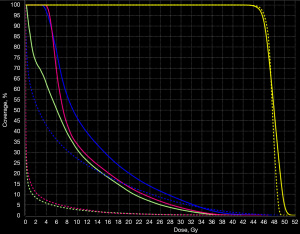
Expander case study
Table 4 shows a planning comparison between the three different techniques, VMAT, PBS with shoot around and PBS with “shoot through” techniques for patient #2. Consistent with Table 3, the PBS plans substantially reduce the OAR doses. In this case, the “shoot around” technique was more conformal than the “shoot through” technique and this was reflected in both the OAR doses and robustness. Specifically, the “shoot around” technique is marginally outside our acceptance criteria for a 5 mm setup covering V95% of the CTV-skin_Eval >95% with coverage down to 92%.
Table 4
| Dosimetric parameter | VMAT | Shoot around PBS | Shoot through PBS |
|---|---|---|---|
| CI | 1.17 | 1.09 | 1.10 |
| HI | 1.17 | 1.14 | 1.10 |
| PTV-skin | |||
| D95 [Gy (RBE)] | 45.31 | 45.99 | 46.23 |
| V95 (%Rx) | 99.7 | 99.5 | 99.7 |
| Total lung | |||
| V5 (%) | 88.1 | 38.7 | 32.2 |
| V10 (%) | 47.1 | 26.7 | 20.9 |
| V20 (%) | 20.9 | 13.9 | 10.5 |
| V30 (%) | 7.7 | 5.8 | 4.6 |
| V40 (%) | 0.5 | 1.5 | 1.3 |
| Mean dose [Gy (RBE)] | 13.08 | 7.49 | 6.22 |
| Heart | |||
| Mean dose [Gy (RBE)] | 11.14 | 1.02 | 1.10 |
| V5 (%) | 86.7 | 6.0 | 6.7 |
| V20 (%) | 15 | 0.72 | 0.41 |
| Thyroid mean dose [Gy (RBE)] | 21.3 | 18.6 | 17.6 |
| Liver mean dose [Gy (RBE)] | 8.41 | 0.84 | 0.87 |
| Esophagus max dose [Gy (RBE)] | 46.27 | 41.41 | 36.49 |
VMAT, volumetric arc therapy; PBS, pencil beam scanning; CI, conformality index; HI, homogeneity index; PTV, planning target volume; Rx, prescription.
Discussion
For comparable coverage, proton plans provided better sparing of lungs and heart consistently across nearly all dosimetric parameters. The finite range of the proton beam permits improved sparing of the downstream OARs compared to VMAT. We acknowledge that while we chose patients to represent a cross section of whole breast, chest wall and expander patients, our selection, along with the small sample size may bias our results. Regardless, the study is limited by small patient population studied and while modest, is suggestive. We hope to collect larger number of patients for a future study that would improve the statistical power.
There was no difference in conformality of the plans as expected since all plans achieved good coverage, however the homogeneity was poorer on the VMAT plans since photon plans typically have greater hot spots than proton plans. Part of the variation reflects the challenges of creating a clinically realistic plan. For patient #4, coverage from the VMAT plan was compromised to keep lung V20 <30% for a clinically acceptable plan (data not shown). In this case, CTV V95-skin was 95.9% whereas it was approximately 100% for other plans. However, this small change will not materially change our conclusions.
Our results are in good agreement with the publication by Jimenez et al. (5), although our methods are slightly different. Their photon comparison was using 3D conformal tangents whereas ours used VMAT, reflecting technological advancements in treatment techniques. All their patients had implants where the patient cohort in this study was heterogeneous. Despite the fact that our patient population is a mix of expanders, whole breast and chest wall patients, we found that there are significant improvements in heart and lung sparing with PBS and improved homogeneity compared to VMAT plans. We did not include patients who had implants, but they likely would not affect our findings as they are very similar to expander patients, with the primary differences between the absence of the magnet and housing and potentially, silicone filling compared to saline which must be accounted for in the planning process (20).
Our results also agree with those of Vyfhuis et al. (6) who presented three different cases and 3 different planning approaches. Part of their planning decisions were influenced by the patient size, planning and delivery times, as well as OAR robustness. We used a universal approach with two isocenters and a multiple SFUD fields employing an MFO match only in the overlap region between the two breasts. We found very good robustness and reasonable delivery times.
Our results also agree with those of Sung et al. (1). Specifically, a comparison PBS and VMAT showed PBS plans reduced the dose to heart and lung metrics. There are minor differences, however. For instance, our lung V40 is not significantly different between PBS and VMAT plans whereas theirs was, but in both our data and their data, the differences are approximately 2–3% and the statistical significance is a measure of how tightly bunched the data is and is affected by our relatively smaller sample size.
The presence of an expander did not affect the results dramatically. The shoot through approach to treating the expander yielded very similar distributions to patients with intact breasts. Moreover, the differences between the PBS and VMAT plans follow the same trends as the other patients, however a larger patient sample set is required to verify this conclusion.
We chose to perform our plans on free breathing patients. It has been demonstrated that use of DIBH reduces the lower doses to OARs (e.g., lung V20, mean heart dose) for unilateral breast cancers by providing a greater separation between the breast and these OARs (21). The relative gains from DIBH may be estimated by considering the results of previous investigations. Dumane et al. (18) reported a reduction of the mean heart dose from 8.2 to 5.3 Gy using DIBH and photons. Table 3 shows our mean VMAT heart dose was 11.1 Gy while the PBS mean heart dose was 0.81 Gy (RBE). There is a greater than factor of 10 reduction in mean heart dose for PBS and a factor of <2 for DIBH. Similarly, the mean free breathing lung V20 was 13.1 Gy was reduced to 12.4 Gy. In comparison, our free breathing VMAT V20 of 15 Gy was reduced to 0.65 Gy (RBE) with PBS.
This study focused on a retrospective analysis of proton plans and used the proton PTVs which may not be identical to photon PTVs. The most significant difference is likely the expansion of the photon fluence to account for respiratory motion in the anterior posterior dimension. For protons, this margin is not necessary as the dosimetric variation for an en face beam approximately 2 m away is approximately 1% per cm of motion (due to inverse square law) and hence negligible for most patients. In the photon plans, this PTV expansion may not be sufficient to account for respiration hence the values here may be considered conservative in terms of gains that may come from a proton plan.
While it has been reported that doses to specific vessels may be better metrics for risk, we were unable to accurately delineate these without a contrast CT. However, the mean heart dose is dramatically different and is correlated with subsequent cardiac events (22) and is the chosen metric for a large on-going clinical study (23).
Conclusions
In agreement with previous investigators, we have found that PBS proton plans provide better sparing of organs at risk particularly for lower doses than VMAT. PBS can significantly spare lung, heart and liver. We have included a bilateral expander patient using two different planning techniques, one which treats through the magnet and the other that treats around it, but the results are not very sensitive to the technique. Comparison of our relative gains to the literature suggest that gains from protons are more substantial than gains from DIBH, but we caution that this needs to be verified.
Acknowledgments
Funding: This work was supported by the MSK Institutional Core Grant (No. NCI P30 CA008748).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Therapeutic Radiology and Oncology for the series “Pencil Beam Scanning Particle Therapy”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tro.amegroups.com/article/view/10.21037/tro-22-23/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tro.amegroups.com/article/view/10.21037/tro-22-23/coif). The series “Pencil Beam Scanning Particle Therapy” was commissioned by the editorial office without any funding or sponsorship. D.M. served as the unpaid Guest Editor of the series. Laura Happersett, Linda Hong and Seng Boh Lim are employees of Memorial Sloan Kettering Cancer Center and declare NIH/NCI Cancer Center Support Grant (No. P30 CA008748). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Western IRB (No. PC 2016-01) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Sakai T, Ozkurt E, DeSantis S, et al. National trends of synchronous bilateral breast cancer incidence in the United States. Breast Cancer Res Treat 2019;178:161-7. [Crossref] [PubMed]
- Mejdahl MK, Wohlfahrt J, Holm M, et al. Breast cancer mortality in synchronous bilateral breast cancer patients. Br J Cancer 2019;120:761-7. [Crossref] [PubMed]
- Mutter RW, Choi JI, Jimenez RB, et al. Proton Therapy for Breast Cancer: A Consensus Statement From the Particle Therapy Cooperative Group Breast Cancer Subcommittee. Int J Radiat Oncol Biol Phys 2021;111:337-59. [Crossref] [PubMed]
- Jimenez RB, Goma C, Nyamwanda J, et al. Intensity modulated proton therapy for postmastectomy radiation of bilateral implant reconstructed breasts: a treatment planning study. Radiother Oncol 2013;107:213-7. [Crossref] [PubMed]
- Vyfhuis MAL, Zhu M, Agyepong B, et al. Techniques for Treating Bilateral Breast Cancer Patients Using Pencil Beam Scanning Technology. Int J Part Ther 2019;6:1-11. [Crossref] [PubMed]
- Sun T, Lin X, Tong Y, et al. Heart and Cardiac Substructure Dose Sparing in Synchronous Bilateral Breast Radiotherapy: A Dosimetric Study of Proton and Photon Radiation Therapy. Front Oncol 2019;9:1456. [Crossref] [PubMed]
- RADCOMP Breast Atlas. USA: NRG Oncology. Available online: https://www.nrgoncology.org/About-Us/Center-for-Innovation-in-Radiation-Oncology/Breast/RADCOMP-Breast-Atlas
- Cooper BT, Goenka A, Sine K, et al. Development of a Comprehensive, Contour-Based, Peer Review Workflow at a Community Proton Center. Int J Part Ther 2020;7:34-40. [Crossref] [PubMed]
- DeCesaris CM, Rice SR, Bentzen SM, et al. Quantification of Acute Skin Toxicities in Patients With Breast Cancer Undergoing Adjuvant Proton versus Photon Radiation Therapy: A Single Institutional Experience. Int J Radiat Oncol Biol Phys 2019;104:1084-90. [Crossref] [PubMed]
- Paganetti H, Niemierko A, Ancukiewicz M, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys 2002;53:407-21. [Crossref] [PubMed]
- Pankuch M, Gao M, Gans S, et al. A Novel Proton Therapy Technique for Treatment of Postmastectomy Breast Cancer Patients With Tissue Expanders Containing High-Density Metallic Filling Ports. Int J Radiat Oncol Biol Phys 2016;96:E689. [Crossref]
- Kirk M, Freedman G, Ostrander T, et al. Field-Specific Intensity-modulated Proton Therapy Optimization Technique for Breast Cancer Patients with Tissue Expanders Containing Metal Ports. Cureus 2017;9:e1698. [Crossref] [PubMed]
- Zhu M, Langen K, Nichols EM, et al. Intensity Modulated Proton Therapy Treatment Planning for Postmastectomy Patients with Metallic Port Tissue Expanders. Adv Radiat Oncol 2022;7:100825. [Crossref] [PubMed]
- Mutter RW, Remmes NB, Kahila MM, et al. Initial clinical experience of postmastectomy intensity modulated proton therapy in patients with breast expanders with metallic ports. Pract Radiat Oncol 2017;7:e243-52. [Crossref] [PubMed]
- Zheng Y, Kang Y, Zeidan O, et al. An end-to-end assessment of range uncertainty in proton therapy using animal tissues. Phys Med Biol 2016;61:8010-24. [Crossref] [PubMed]
- Bernstein MB, Walker K, Gillespie E, et al. Bilateral Regional Nodal Irradiation Using Volumetric Modulated Arc Therapy: Dosimetric Analysis and Feasibility. Pract Radiat Oncol 2022;12:189-94. [Crossref] [PubMed]
- Dumane VA, Saksornchai K, Zhou Y, et al. Reduction in low-dose to normal tissue with the addition of deep inspiration breath hold (DIBH) to volumetric modulated arc therapy (VMAT) in breast cancer patients with implant reconstruction receiving regional nodal irradiation. Radiat Oncol 2018;13:187. [Crossref] [PubMed]
- Feuvret L, Noël G, Mazeron JJ, et al. Conformity index: a review. Int J Radiat Oncol Biol Phys 2006;64:333-42. [Crossref] [PubMed]
- Moyers MF, Mah D, Boyer SP, et al. Use of proton beams with breast prostheses and tissue expanders. Med Dosim 2014;39:98-101. [Crossref] [PubMed]
- Nissen HD, Appelt AL. Improved heart, lung and target dose with deep inspiration breath hold in a large clinical series of breast cancer patients. Radiother Oncol 2013;106:28-32. [Crossref] [PubMed]
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. [Crossref] [PubMed]
- Pragmatic Randomized Trial of Proton vs. Photon Therapy for Patients With Non-Metastatic Breast Cancer: A Radiotherapy Comparative Effectiveness (RADCOMP) Consortium Trial. NCT02603341.
Cite this article as: Mah D, Happersett L, Del Rosario G, Lim SB, Hong L. A retrospective bilateral breast proton pencil beam scanning and photon volumetric arc therapy planning comparison. Ther Radiol Oncol 2023;7:16.


