
Long term-control via successful salvage therapy with cytoreductive surgery, chemotherapy, and radiotherapy for recurrence with intraperitoneal carcinomatosis of synchronous advanced ovarian and endometrial cancer: a case report
Highlight box
Key findings
• This case report is the first to describe a rare case of synchronous endometrioid carcinomas of the uterus and ovaries (SEOCs) complicated with advanced-stage, recurrent clear cell carcinoma and peritoneal carcinomatosis.
What is known and what is new?
• Synchronous endometrioid carcinomas of the uterus and ovaries are rare.
• The transformation to a clear cell carcinoma on recurrence is a unique feature of our case. This case is a long-term survivor of recurrent SEOC with peritoneal clear cell carcinomatosis.
What is the implication, and what should change now?
• Our findings have important implication on multidisciplinary treatments for recurrent synchronous ovarian and endometrial cancers that may help to define optimal treatment regimens and improve patient care and survival.
Introduction
Synchronous endometrioid carcinomas found both in the uterus and ovaries are uncommon but well recognized (1-3). Synchronous endometrial and ovarian carcinomas (SEOCs) may be two distinct primary tumors, or one may be a metastasis from the endometrium or the ovaries (2). The diagnosis of SEOCs requires careful examination that considers gross tumor laterality, histological features, and endometriosis status (1-3). Not only is diagnosis difficult; the treatments are challenging. The adjuvant radiotherapies for endometrial and ovarian cancers are quite different. Radiotherapy has been suggested for high-risk endometrial cancers, but not for ovarian cancers. The lack of any benefit on addition of radiotherapy for patients with ovarian cancer suggests that adjuvant radiotherapy plays only a palliative role. Thus, a treatment dilemma arises when considering an initial treatment for synchronous advanced ovarian and early endometrial cancers; this becomes even more difficult if disease recurs.
Most studies reported a favorable outcome if SEOCs were detected early and were of one of the common histological types, such as endometrioid carcinoma (3,4). In the largest retrospective cohort of SEOCs, more than 80% of all cancers were of the endometroid cell type, and the rest endometrioid/clear cell subtypes (4). The incidence of synchronous SEOCs was approximately 3.9–10% in patients with stage IA endometrial carcinomas; synchronous ovarian carcinomas evidenced no significant effects on survival (4-6). No study has yet described treatments for advanced-stage SEOCs. Clear cell carcinomas constitute approximately 5% of all primary ovarian cancer and are much more aggressive than other epithelial-type carcinomas (7). The 10-year disease-free survival rates of patients with primary, ovarian clear carcinomas was less than 15%, despite clinical remission prior to recurrence (7).
This case report is the first to describe a rare case of SEOC complicated with advanced-stage, recurrent clear cell carcinoma and peritoneal carcinomatosis. To the best of our knowledge, no long-term survivor of recurrent SEOC with peritoneal clear cell carcinomatosis has been reported. Here, we describe a patient who received a second cytoreductive surgery, salvage chemotherapy, and radiotherapy and achieved long-term control after experiencing intraperitoneal carcinomatosis caused by clear cell carcinoma in accordance with the CARE reporting checklist (available at https://tro.amegroups.com/article/view/10.21037/tro-22-35/rc).
Case presentation
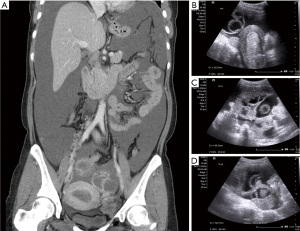
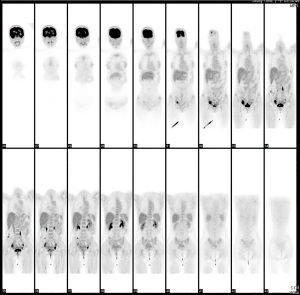
A 39-year-old nulliparous woman with a body mass index of 20.2 kg/m2 visited our emergency department in 2013 with the complaints of progressive abdominal distension (present for 1 week) and vaginal bleeding (present for 2 weeks). She had a history of laparotomy; her spleen had ruptured after a severe traffic accident 2 years prior. She had a history of hypertension and hepatitis B infection, although she denied diabetes mellitus and coronary artery disease. Her menstrual cycle had originally been 28 days in duration, while her period had lasted for 7 days. At the time of the emergency department visit, her menstrual cycle was approximately 2 months in duration, while her period lasted for 16 days. She denied the use of any exogenous hormone. Her grandfather had died of lung cancer but she reported no other family history of cancer. Computed tomography (CT) and ultrasonography revealed bilateral complex ovarian tumors of ≤9.6 cm in diameter, a thick endometrium (≤29.3 mm), massive ascites, and substantial intraperitoneal tumor seedings (Figure 1A-1D). Her initial serum levels of cancer antigen 125 (CA-125) and cancer antigen 19-9 (CA19-9) were 595 and 54 U/mL, respectively. Histological examination of an endometrial sample revealed high-grade adenocarcinoma. Thus, laparotomy was performed. Intraoperative findings included 8,500 mL of bloody ascites; a ruptured, right ovarian complex tumor 12 cm in diameter; a left ovarian complex tumor 8 cm in diameter; multiple peritoneal nodules, including an omental nodule 2 cm in diameter; a left paravesical area of 8 cm2; a bladder serosal area of 2 cm2; a paracolic gutter 1 cm in depth; and a small intestinal mesenteric lesion 1 cm in length. The peritoneal cancer index was 16. Optimal debulking surgery (total hysterectomy, bilateral salpingo-oophorectomy, bilateral pelvic lymph node dissection, omentectomy, and peritoneal tumor resection) removed all macroscopic disease. Pathology revealed grade 3 endometrioid carcinoma of the endometrium with 5% myometrial invasion and the absence of lymphovascular permeation; grade 3 endometrioid carcinomas of both ovaries; and metastatic peritoneal tumors. Endometrial tissue was present in the appendix. The final diagnosis was synchronous stage IA endometrial cancer and stage IIIC ovarian cancer. The tumor expressed the estrogen receptor (ER) (6f11) (95% of cells strongly positive) and the progesterone receptor (PR) (1a6) (3% of cells intermediately positive). A complete response was achieved after six cycles of adjuvant dose-dense intravenous chemotherapy (paclitaxel and carboplatinum). However, elevated CA-125 and CA19-9 levels were noted 22 months after the last course of chemotherapy in Feb 2016. The diagnostic results from CT scan showed recurrent cystic mass on left iliac wall and suspected recurrent peritoneal carcinomatosis on Feb/15/2016. Positron emission tomography (PET) revealed peritoneal seedings in the pelvic regions, both paracolic gutters, and soft tissue posterior to the right psoas muscle; a subcapsular nodule was present over the S6 liver surface (all scores 4= high probability of malignancy, Figure 2) on Feb/24/2016. Cytoreductive surgery was performed; extensive peritoneal tumors were noted (peritoneal cancer index =22). After extensive tumor resection, the completeness of cytoreduction score was 1; thin residual miliary lesions near the left external iliac vessels were indicated using hemoclips. An intraperitoneal port was placed. Histological examination revealed recurrent ovarian clear cell carcinomas (all peritoneal tumors). Immunohistochemical analysis revealed expression of MLH1 (GM011), MSH2 (G219-1129), MSH6 (EPR3945), and PMS2 (A16-4). ER expression was weaker in recurrent tumors than in the initial tumors (20% of cells stained moderately); recurrent tumors lacked PR expression.
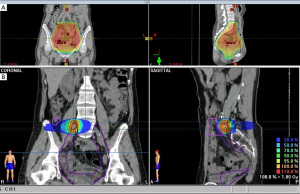
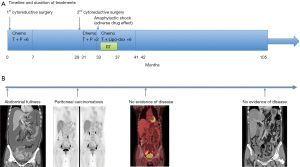
Adjuvant intraperitoneal chemotherapy [carboplatin AUC 6 on day 1 and intravenous chemotherapy with paclitaxel 80 mg/m2 on days 1 and 15 (two cycles)] was commenced; however, carboplatin was switched to intravenous liposomal doxorubicin (Lipo-dox) (40 mg/m2) for the subsequent six cycles because of anaphylactic shock (Naranjo score 6). A grade 4 hematological adverse event developed; thus, the chemotherapy dose was adjusted to 75%. The CA-125 and CA19-9 levels remained high and a radiation oncologist was consulted regarding salvage radiotherapy. This treatment was delivered to the entire pelvis (4,500 cGy/25 fractions) with a cone-down boost to the right lower pelvis (up to 5,040 cGy/28 fractions via the box-field technique), followed by a boost of up to 5,940 cGy/33 fractions (via five-field intensity-modulated radiation therapy) to suspected residual lesions in the right external iliac area (Figure 3). Treatment was interrupted for 3 weeks when hematopoietic toxicity developed after the patient had received seven external radiotherapy treatments combined with Lipo-dox and paclitaxel. A complete response was achieved (Figure 4). Today, more than 5 years later, the patient remains disease-free. The patient is followed regularly in every 4–6 months both in Gynecology Oncology and Radiation Oncology Outpatient Department and undergoes regularly scheduled CT and positron-emission tomography examinations. The patient did not suffer from any grade II or more gastrointestinal (GI) or genitourinary (GU) toxicity during the follow-up time. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Patients with recurrent ovarian clear cell carcinomas are less sensitive to platinum-based front-line chemotherapy and have poor prognoses compared to patients with other epithelial-type carcinomas (7,8). We thought it appropriate to change the chemotherapy from carboplatinum to lipodox because our patient evidenced recurrent advanced disease and anaphylactic shock. Clear cell and endometroid carcinomas are the most common histological types of primary ovarian cancers in women with associated endometriosis (9-11). Endometriosis is a benign gynecological disease characterized by endometrial glands and stroma formation outside the uterus; however, it is a risk factor for epithelial ovarian cancer (9-11). The rate of malignant transformation is 0.3–0.8% and the relative risk ranges from 1.3 to 1.9 (9). Endometriotic tissue was found in the appendix of our present this case; however, the lesion might be located in the usual common sites, such as both ovaries. Among patients with primary ovarian malignancies, those lacking endometriosis tend to be significantly older (mean 55 years) than patients with endometriosis elsewhere in the pelvis (mean 45 years) (9,11,12). Endometriosis may have triggered the malignancies in the present patient; she is young, and lacks other risk factors such as obesity, excess hormone exposure, and a family cancer history. Endometriosis-associated primary ovarian cancers are relatively benign in the early stages if the cell type is endometroid (9,11,12). The transformation to a clear cell carcinoma on recurrence is a unique feature of our case. However, for both the initial endometrioid carcinoma and the recurrent clear cell carcinoma, the pathology was comparable to that of endometriosis-related primary ovarian cancer with a common histology.
Another unique feature of this case is that not only the ovarian cancer recurred, both SEOCs recurred. On recurrence, we initially used the treatment strategies earlier employed for advanced ovarian cancer, but then shifted to salvage radiotherapy combined with sequential chemotherapy, unlike the conventional chemotherapeutic strategies for recurrent ovarian cancers. Current National Comprehensive Cancer Network (NCCN) guidelines do not consider adjuvant radiotherapy to be the standard of care for primary ovarian cancer, and any salvage role of radiotherapy for patients with recurrent ovarian cancer has been debated and may be detrimental (13). We used radiotherapy to rescue the patient. We chose this over a second-line chemotherapy regimen or extension of the existing chemotherapy to treat primary advanced ovarian cancer. Two major considerations in shifting the treatment strategy were the known aggressive nature of SECOs and the episode of anaphylactic shock that developed during salvage chemotherapy. The radiotherapy treatment time was prolonged because of the profound bone marrow suppression by the prior chemotherapy. The total dose to the pelvic region was 45 Gy. The suspected residual lesions in the right external iliac area received boosts of up to 59.4 Gy based on the surgical findings and CT simulation of salvage radiotherapy (Figure 3). Suspected residual clear cell carcinomas may require boosting with ≥60 Gy, which was independently prognostic of improved survival in one recent retrospective study (14).
The initial pathology of our case was a poorly differentiated endometroid carcinoma and compatible with the most common histology of SEOCs. However, the clinical behavior was more aggressive than usual, associated with a less common histological type (i.e., clear cell carcinoma). Specific immunohistochemical markers such as p53 and p16, DNA mismatch repair proteins, PTEN, and ARID1A distinguish endometrioid carcinoma from clear cell carcinoma (9,10). Tanase et al. postulated that certain events (e.g., cessation of ER expression) were required for progression of clear cell carcinomas (15). The recurrent tumors of our patient exhibited decreased ER expression (from 95% to 30%) and PR expression (from 3% to 1%) compared to the primary tumors. Also, the recurrent tumors expressed MLH1 (GM011), MSH2 (G219-1129), MSH6 (EPR3945), and PMS2 (A16-4), which indicates that DNA mismatch repair proteins are synthesized by clear cell carcinomas (9,10).
Conclusions
In conclusion, we report successful salvage of recurrent SEOCs. If SEOCs are associated with endometriosis, recurrence may feature a clear cell carcinoma. SEOCs are high-risk endometrial cancers but adjuvant radiotherapy or early salvage radiotherapy may afford long-term control. Based on this case, salvage radiotherapy may assist patients with more aggressive diseases, such as recurrent clear cell carcinomas. The strengths of this case are that the follow-up time is more than 9 years and the disease control status is confirmed. The limitation is that we have no information about the clonally related origin of the recurrent clear cell carcinomas. In our patient, aggressive multidisciplinary treatment of the recurrence (i.e., cytoreductive surgery, intraperitoneal chemotherapy, and salvage radiotherapy) afforded long-term cancer control.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tro.amegroups.com/article/view/10.21037/tro-22-35/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tro.amegroups.com/article/view/10.21037/tro-22-35/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fadare O, Parkash V, Dupont WD, et al. The diagnosis of endometrial carcinomas with clear cells by gynecologic pathologists: an assessment of interobserver variability and associated morphologic features. Am J Surg Pathol 2012;36:1107-18. [Crossref] [PubMed]
- Chao A, Wu RC, Jung SM, et al. Implication of genomic characterization in synchronous endometrial and ovarian cancers of endometrioid histology. Gynecol Oncol 2016;143:60-7. [Crossref] [PubMed]
- de la Fouchardière A, Frachon S, Gengler C, et al. Independent endometrial and ovarian carcinomas: two cases of synchronous and metachronous endometrioid carcinomas. Ann Pathol 2004;24:172-5. [PubMed]
- Zhan X, Li L, Wu M, et al. The prognosis of stage IA synchronous endometrial endometrioid and ovarian carcinomas. Arch Gynecol Obstet 2019;300:1045-52. [Crossref] [PubMed]
- AlHilli MM, Dowdy SC, Weaver AL, et al. Incidence and factors associated with synchronous ovarian and endometrial cancer: a population-based case-control study. Gynecol Oncol 2012;125:109-13. [Crossref] [PubMed]
- Zaino R, Whitney C, Brady MF, et al. Simultaneously detected endometrial and ovarian carcinomas—a prospective clinicopathologic study of 74 cases: a gynecologic oncology group study. Gynecol Oncol 2001;83:355-62. [Crossref] [PubMed]
- Chan JK, Teoh D, Hu JM, et al. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol 2008;109:370-6. [Crossref] [PubMed]
- Zhou L, Yao L, Dai L, et al. Ovarian endometrioid carcinoma and clear cell carcinoma: A 21-year retrospective study. J Ovarian Res 2021;14:63. [Crossref] [PubMed]
- Murali R, Davidson B, Fadare O, et al. High-grade Endometrial Carcinomas: Morphologic and Immunohistochemical Features, Diagnostic Challenges and Recommendations. Int J Gynecol Pathol 2019;38:S40-63. [Crossref] [PubMed]
- Worley MJ Jr, Liu S, Hua Y, et al. Molecular changes in endometriosis-associated ovarian clear cell carcinoma. Eur J Cancer 2015;51:1831-42. [Crossref] [PubMed]
- Noli S, Cipriani S, Scarfone G, et al. Long term survival of ovarian endometriosis associated clear cell and endometrioid ovarian cancers. Int J Gynecol Cancer 2013;23:244-8. [Crossref] [PubMed]
- Wei JJ, William J, Bulun S. Endometriosis and ovarian cancer: a review of clinical, pathologic, and molecular aspects. Int J Gynecol Pathol 2011;30:553-68. [Crossref] [PubMed]
- Herrera FG, Irving M, Kandalaft LE, et al. Rational combinations of immunotherapy with radiotherapy in ovarian cancer. Lancet Oncol 2019;20:e417-33. [Crossref] [PubMed]
- Yang H, Zhang K, Liu Z, et al. Clinical analysis of conformal and intensity-modulated radiotherapy in patients with recurrent ovarian cancer. Sci Rep 2020;10:17172. [Crossref] [PubMed]
- Tanase Y, Yamada Y, Shigetomi H, et al. Modulation of estrogenic action in clear cell carcinoma of the ovary Exp Ther Med 2012;3:18-24. (Review). [Crossref] [PubMed]
Cite this article as: Lin SM, Tang YH. Long term-control via successful salvage therapy with cytoreductive surgery, chemotherapy, and radiotherapy for recurrence with intraperitoneal carcinomatosis of synchronous advanced ovarian and endometrial cancer: a case report. Ther Radiol Oncol 2023;7:21.


