Analyses of treatment outcomes in advanced vulvar cancer: treated with postoperative radiotherapy or definitive radiotherapy/chemoradiotherapy
Highlight box
Key findings
• For patients with stage III–IVA vulvar squamous cell carcinoma (SCC) treated with radiotherapy (RT)/chemoradiotherapy (CRT), the 2-year overall survival and disease-free survival were 59.7% and 43.9%, respectively.
• Most patients suffered from grade 1–2 skin (83.3%) and genitourinary (61.1%) acute reactions.
What is known and what is new?
• Many studies revealed a therapeutic benefit with CRT in advanced vulvar cancer.
• We provide the survival outcomes and the toxicity profile of patients with advanced vulvar cancer treated with postoperative radiotherapy (PORT) and definitive RT/concurrent chemoradiotherapy (CCRT).
What is the implication, and what should change now?
• For stage III–IVA vulvar SCC, both PORT and definitive RT/CCRT are reasonable options according to the clinical status.
• Skin and genitourinary toxicities should be monitor carefully during the treatment.
Introduction
Vulvar cancer is a rare gynecologic malignancy, accounting for five percent of all gynecologic cancers, with an incidence of 2.5 per 100,000 women in America (1), while in Taiwan the corresponding figures are 3.1% and 1.48 per 100,000 women, respectively (2). In the United States, 90% of patients with vulvar cancer are diagnosed with in-situ or early-stage invasive disease, while older patients are more likely to present with an advanced disease (3). Surgery is the major management for vulvar cancer. Dissection of bilateral inguino-femoral and pelvic lymph nodes (LNs) can be performed with radical vulvectomy to improve overall survival (OS), but the procedure can result in morbidities, such as wound disruption, infection, chronic lymphedema, urinary, or sexual dysfunction (4,5). Radiotherapy (RT) is often used as an adjuvant therapy or for palliative intent. Indications for postoperative radiotherapy (PORT) are positive LN and close/positive surgical margins (6-8).
Many studies revealed a therapeutic benefit with chemoradiotherapy (CRT) in advanced vulvar cancer. Landrum et al. reported no differences in OS and progression-free survival (PFS) between patients treated with primary CRT or primary surgery (9). The prospective phase II trial, the Gynecologic Oncology Group (GOG) 101, enrolled 71 patients with T3/T4 tumors and treated them with two cycles of 5-fluorouracil (5-FU) and cisplatin with RT, to be followed by surgical resection. Thirty-four of 71 patients (48%) had complete response, and 70% of those had no residual tumor in histologic specimens. Preoperative CRT for advanced vulvar cancer may reduce the need for more radical surgery, including primary pelvic exenteration (10). Another phase II trial, GOG 205, enrolled 58 patients with unresectable T3–4 tumors treated with RT (1.8 Gy daily × 32 fractions = 57.6 Gy) plus weekly cisplatin (40 mg/m) followed by surgical resection of residual tumor (or biopsy to confirm complete clinical response). The result yielded high complete clinical and pathologic response rates (37/58; 64%) with acceptable toxicity (11). In 2012, Lee et al. retrospectively compared treatment outcomes between concurrent chemoradiotherapy (CCRT) and PORT. The outcome of patients with vulvar cancer treated with RT showed relatively good local control and low recurrence (12).
The purpose of this study was to investigate the treatment outcomes of patients with locally advanced [International Federation of Gynecology and Obstetrics (FIGO) stage III–IV] vulva cancer in our hospital and to analyze the outcomes of PORT and definitive RT/CCRT.
Methods
Patients
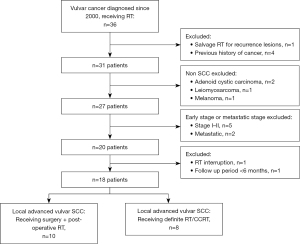
From March 2001 to July 2019, thirty-six patients with vulvar cancer were documented at the radiation oncology department of Taichung Veterans General Hospital, Taiwan. The inclusion criteria were (I) pathologically proven squamous cell carcinoma (SCC) of the vulva; (II) FIGO stage III–IVA, and IVB with only pelvic LN metastasis; (III) patients receiving PORT or definitive RT/CCRT. The exclusion criteria were (I) evidence of distant metastasis at diagnosis (FIGO stage IVB, except only pelvic LN metastasis); (II) patients who received an incomplete treatment course; (III) patients with history of other cancers; and (IV) follow-up period of less than six months. Eighteen patients were finally eligible for inclusion in the study (Figure 1). All patients underwent staging workup, including comprehensive medical history, clinical physical examination, biopsy of vulvar tumor, chest X-ray, diagnostic abdomen and pelvic computed tomography (CT) scan, complete blood cell count, and serologic evaluation of liver and renal functions. Tumor staging was defined according to the FIGO staging system. Written informed consent was obtained from each patient before treatment. All hospital charts, images, and RT records were reviewed.
Surgery
All patients in our study were evaluated by experienced gynecologic oncologists at our hospital, and resectable patients received surgery with radical vulvectomy to achieve 2-cm tumor-free margins plus bilateral inguino-femoral LN dissection. Ten patients with inadequate surgical margins or positive inguinal LNs were referred for PORT. Eight patients with unresectable tumors or deemed a poor candidate for surgery due to old age or poor performance status were referred to a radiation oncologist for definitive RT or CCRT.
RT
All patients were scheduled to undergo external beam radiotherapy (EBRT), and four patients received intracavitary brachytherapy after EBRT. Different radiation treatment techniques were used, including 2D, 3D, and intensity-modulated radiation therapy (IMRT). The contrast-enhanced CT images were obtained to define the vulvar lesion and inguinal LNs. For PORT, the initial field of clinical target volume (CTV) covered the surgical tumor bed plus 0.5 cm margin, and the inguinal LN area was included in the CTV if the pathology report showed positive lymphadenopathy. An initial dose of 4,500 cGy was given, and then a boost dose up to a total of 6,000–7,000 cGy was given to the tumor bed area and positive inguinal LN area. The final RT dose ranged from 4,500 to 7,200 cGy (median dose: 5,700 cGy). For definite RT/CCRT, gross tumor volume (GTV) was defined as the vulvar tumors, and GTV-N was defined as inguinal LNs ≥0.8 cm. The initial field of CTV covered GTV plus 0.5 cm margin, GTV-N plus 0.5 margin, and the involved inguinal LNs area. The planned target volume with a 0.5–1 cm margin superiorly, inferiorly, and radially was given to the CTV. An initial dose of 4,500 cGy was given, and then a boost dose up to a total of more than 7,000 cGy was given to the primary tumor and the involved LNs. The final RT dose ranged from 7,000 to 9,940 cGy (median dose: 7,400 cGy).
Chemotherapy
Four of 18 patients received 6–7 doses of cisplatin 30 mg/m2 per week concurrently with RT. One patient received two courses of cisplatin 75 mg/m2 on day one plus 5-FU 1,000 mg/m2 on days 1–4 per cycle, for four weeks per cycle. Complete blood count test was performed weekly. If the absolute neutrophil count was less than 500/mm3 or the platelet count was less than 100,000/mm3, the chemotherapy was delayed or interrupted until recovery. Dose modifications were prescribed for subsequent cycles based on toxicity grade. If a patient had grade 3–4 hematological toxicity, chemotherapy was held.
Endpoints
The endpoints were OS, local regional relapse-free survival (LRRFS), and distant metastasis relapse-free survival (DMRFS). OS was defined as the time between the date of pathological diagnosis and the date of death or the last contact. LRRFS and DMRFS were defined as the time between the date of pathological diagnosis to the date of recurrence or distant metastasis events detected or the last date of clinical follow-up.
Statistical analysis
The OS, LRRFS, and DMRFS were calculated using the Kaplan-Meier method. All statistical analyses were performed with SPSS 23 (IBM Co., Armonk, NY, USA). A P value less than 0.05 was considered statistically significant. Acute toxicity of RT or chemotherapy was assessed weekly using the Radiation Therapy Oncology Group (RTOG) grading system.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the local Institutional Review Board (IRB No. CE20095A).
Results
The patients’ characteristics are summarized in Table 1. The patients’ ages ranged from 41 to 87 years and the median age was 67 years. There were 14 patients with FIGO stage III disease, and four with FIGO stage IVA and stage IVB with only pelvic LN metastatic disease. Ten patients received PORT, and eight patients received definitive RT/CCRT. Three patients received salvage surgery after definitive RT/CCRT due to residual tumors. A comparison of patients receiving PORT and definitive RT/CCRT showed no significant differences in patients’ age, ECOG score, clinical stage, tumor grading, and RT treatment technique. However, patients in the definitive RT/CCRT group tended to receive higher radiation doses (P=0.0005) and had a higher proportion of receiving concurrent chemotherapy (P=0.0430). Only 1 patient (10%) received concurrent chemotherapy in the PORT group, compared with 5 patients (62.5%) in the definitive RT/CCRT group.
Table 1
| Characteristics | Surgery + PORT (n=10), N (%) | Definitive RT/CCRT (n=8), N (%) | P value |
|---|---|---|---|
| Age (years) | 0.1220 | ||
| Range | 41–74 | 42–87 | |
| Median | 58.5 | 74.5 | |
| Mean ± SD | 60.3±11.2 | 70.0±15.1 | |
| ECOG score | >0.99 | ||
| 0 | 9 (90.0) | 8 (100.0) | |
| ≥1 | 1 (10.0) | 0 (0.0) | |
| T stage | 0.2070 | ||
| 1 | 3 (30.0) | 0 (0.0) | |
| 2 | 6 (60.0) | 6 (75.0) | |
| 3 | 1 (10.0) | 2 (25.0) | |
| N stage | 0.0925 | ||
| 0 | 0 (0.0) | 2 (25.0) | |
| 1 | 5 (50.0) | 2 (25.0) | |
| 2 | 5 (50.0) | 2 (25.0) | |
| 3 | 0 (0.0) | 2 (25.0) | |
| FIGO stage | 0.2745 | ||
| 3 | 9 (90.0) | 5 (62.5) | |
| 4 | 1 (10.0) | 3 (37.5) | |
| Biopsy pathology | 0.2317 | ||
| SCC, WD | 0 (0.0) | 1 (12.5) | |
| SCC, MD | 4 (40.0) | 5 (62.5) | |
| SCC, PD | 6 (60.0) | 2 (25.0) | |
| Neoadjuvant CT before OP or RT | >0.99 | ||
| Yes | 2 (20.0) | 1 (12.5) | |
| No | 8 (80.0) | 7 (87.5) | |
| RT technique | 0.5049 | ||
| 2D | 2 (20.0) | 3 (37.5) | |
| 2D + 3D CRT | 2 (20.0) | 1 (12.5) | |
| 3D | 4 (40.0) | 1 (12.5) | |
| IMRT | 2 (20.0) | 3 (37.5) | |
| RT highest dose | 0.0005 | ||
| Range (cGy) | 4,500–7,020 | 7,000–9,940 | |
| Median (cGy) | 5,700 | 7,400 | |
| Mean ± SD (cGy) | 5,732±836.1 | 7,717.5±994.6 | |
| RT concurrent with CT | 0.0430 | ||
| With concurrent CT | 1 (10.0) | 5 (62.5) | |
| w/o concurrent CT | 9 (90.0) | 3 (37.5) |
PORT, postoperative radiotherapy; RT, radiotherapy; CCRT, concurrent chemoradiotherapy; SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecology and Obstetrics; SCC, squamous cell carcinoma; WD, well differentiated; MD, moderate differentiated; PD, poor differentiated; CT, chemotherapy; OP, operation; 3D CRT, 3D conformal radiotherapy; IMRT, intensity-modulated radiation therapy; w/o, without.
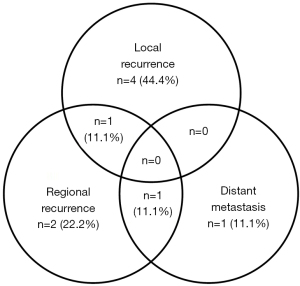
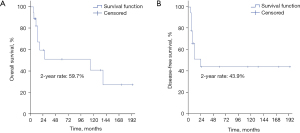
In our study, the median follow-up period for all patients was 16 months, with a range from 6 to 194 months. Ten patients survived less than 2 years after treatment, while the other 8 patients had long-term survival of more than 2 years. For those who survived more than 2 years, the medium follow-up time was 123.5 months. The 2-year OS and DFS were 59.7% and 43.9%, respectively (Figure 2A,2B). There were nine recurrences (four local alone, two regional alone, one distant metastasis alone, one local plus regional metastasis, one regional plus distant metastasis) (Figure 3), and 9 deaths (50%).
Among the 10 patients who received surgery, two patients suffered from poor wound healing and recovered after antibiotic treatment and wound care. One patient had a hematoma that formed at the inguinal area and received debridement. The 2-year OS, LRRFS, and DMRFS were 53.3%, 58.3%, and 65.6%, respectively.
Of the 8 patients who received definitive RT/CCRT, five patients (62.5%) achieved complete response after receiving definitive RT/CCRT, and the other three patients with residual tumor received salvage surgery. The 2-year OS, LRRFS, and DMRFS were 66.7%, 45.0%, and 100%, respectively.
The numbers of patients with acute toxicities are summarized in Table 2. In all patients (n=18), most patients suffered from grade 1–2 skin (83.3%) and genitourinary (61.1%) reactions. Grade three skin reactions were found in 3 patients (16.7%), and grade three hematological toxicity was found in another 3 patients (16.7%). Late toxicities in the PORT group included cellulitis in 2 patients. Late toxicities in the RT/CCRT group included cellulitis in one patient, skin necrosis and poor healing in 4 patients, and proctitis in one patient.
Table 2
| RTOG grade | Acute complication | ||
|---|---|---|---|
| I–II | III | IV | |
| Hematological | 3 | 3 | 0 |
| Skin | 15 | 3 | 0 |
| Genitourinary | 11 | 0 | 0 |
| Gastro-intestinal | 5 | 0 | 0 |
RTOG, Radiation Therapy Oncology Group.
Discussion
This retrospective study assessed outcomes in patients with stage III–IVA vulvar SCC treated by PORT or definitive RT/CCRT. The 2-year OS and DFS were 59.7% and 43.9%, respectively. A systematic review in 2016 by Zhou et al. (13) showed the pooled 5-year OS rate of stage I–IV vulva cancer was 64.9%, and patients with FIGO III and IV disease had 5-year OS rates of 47.8% and 9.4%, respectively. The 5-year DFS rate was 87.2% for patients with no LN metastasis and for patients with ≥3 LNs metastases the rate was 35.4%. Another analysis by Tanaka et al. (14) reported that the 5-year OS in 2001–2008 for localized disease, regional LN invasion, involvement of an adjacent organ, and distant metastases were 81.1%, 32.9%, 27.0%, and 20.8%, respectively. Furthermore, a high recurrence rate was noted in a previous study, with an estimated annual local recurrence rate of 4% without plateauing (15). Our data are comparable with the above studies.
Traditionally, RT serves as either PORT or palliative therapy for recurrent disease. Kunos et al. reported the long-term outcome and toxicities of postoperative inguinal-pelvic RT (45–50 Gy) compared with pelvic node resection (GOG 37). The cancer-related death rate was significantly higher for pelvic node resection compared with radiation (51% vs. 29% at 6 years, P=0.015). RT improved the six-year survival in patients with clinically suspected or fixed ulcerated groin nodes (P=0.004) and two or more positive groin nodes (P<0.001). PORT significantly reduced local relapses and decreased cancer-related deaths (16).
Definitive RT/CCRT also showed an acceptable treatment response. Stecklein et al. (17) in 2018 reported that 407 patients who received definitive RT (median dose to gross lesion, 66.0 Gy) had a 3-year OS rate of 51%. The three-year actuarial incidences of vulvar, groin, and distant recurrences were 24.2%, 17.7%, and 30.3%, respectively. Another study (18) in 2016 evaluated treatment outcomes of definitive CCRT in vulvar cancer. The 3-year OS was 71%, freedom from local recurrence was 65%, and freedom from distant recurrence was 78%. Comparing CCRT to RT, Rao et al. (19) in 2017 reported that CCRT was associated with a reduced hazard of death compared to RT [hazard ratio (HR): 0.76 (0.63–0.91), P=0.003]. The effect remained significant after propensity score matching [HR: 0.78 (0.63–0.97), P=0.023]. In our study, the 2-year OS was 66.7% in the definitive RT/CCRT group. No survival difference was noted between RT and CCRT (P=0.1966), and the reason could be the small sample size in each subgroup.
Among nine patients with disease relapse in our study, only two patients had distant metastases. Loco-regional failure is the main recurrence pattern for vulvar cancer. Vorbeck et al. (20) in 2019 evaluated 157 patients who received RT for vulvar cancer. Patients who received definitive RT developed failure primarily in the high-dose region (80.5%), whereas patients who received PORT had a more scattered failure pattern (P<0.0001). A similar pattern was found in our study. Among 10 patients who received PORT in our study, there was one local plus regional recurrence, two regional recurrence alone, one regional plus distant relapse, and one distant relapse alone. Moreover, four out of eight patients who received definitive RT/CCRT had local recurrence. No patients developed distant metastasis in the definitive RT/CCRT group. A higher proportion of patients in the definitive RT/CCRT group received concurrent systemic chemotherapy, which may explain the better distant control and OS that was achieved. However, the toxicity of RT usually limits the dose escalation at the primary tumor, and local recurrence could be a problem in both RT and CCRT. Close monitoring and biopsy for suspicious lesions at the primary site should be performed after definitive RT/CCRT.
Acute skin complications were mostly seen during RT for vulvar cancer, and patients sometimes needed a break due to severe skin reactions. Lee et al. (12) observed 24 patients who received RT for vulvar cancer, and all of them suffered from acute skin complications (12 patients with grade I–II skin reactions, 11 patients with grade III, and one patient with grade IV). Another study (21) showed 45 out of 56 patients who received curative RT or CCRT had acute skin complications (14 patients with grade I, 18 patients with grade II, and 12 patients with grade III). In our study, all patients had acute skin reactions (15 patients with grade I–II, three patients with grade III). Two patients took a break during RT due to severe skin reactions. Good supportive care for acute skin toxicity may prevent treatment interruption.
There were some limitations in this retrospective study. Because vulvar cancer is rare and surgery is the main treatment, only 18 patients were included in this study. The sample size was too small to attain statistical power. Moreover, a median follow-up of 16 months is too short to draw meaningful conclusions about the recurrence rate and survival. In our study, 10 patients survived less than 2 years after treatment, while the other 8 patients had long-term survival of more than 2 years. For those who survived more than 2 years, the medium follow-up time was 123.5 months. Since vulva cancer is a rare disease, more cases are needed to draw a meaningful conclusion in future studies. Furthermore, seventy-two percent of patients in our study received traditional 2D RT or 3D CRT. The toxicity rate may be lower in the era of IMRT. However, our study revealed definitive RT/CCRT was beneficial with respect to OS and distant control. Further studies should be conducted with an emphasis on improving local control and observing toxicities with the IMRT technique.
Conclusions
Vulvar cancer is a rare gynecologic malignancy. Advanced vulvar cancer carries high local recurrence rates and distant metastasis rates. For stage III–IVA vulvar SCC, both postoperative RT and definitive RT are reasonable options according to the clinical status. Skin and genitourinary toxicities should be monitor carefully during the treatment.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tro.amegroups.com/article/view/10.21037/tro-22-28/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the local Institutional Review Board (IRB No. CE20095A). Written informed consent was obtained from each patient before treatment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bethesda MD. SEER cancer statistics factsheets: vulvar cancer. vol. 2016, National Cancer Institute. Available online: https://seer.cancer.gov/statfacts/html/vulva.html (Internet, cited 2019 Sep 27).
- Health Promotion Administration, Ministry of Health and Welfare, Taiwan. Health Promotion Administration Annual Report 2018. Available online: https://www.hpa.gov.tw/Pages/ashx/File.ashx?FilePathZ~/File/Attach/10231/File_11648.pdf. Accessed Sep 27, 2019.
- Stroup AM, Harlan LC, Trimble EL. Demographic, clinical, and treatment trends among women diagnosed with vulvar cancer in the United States. Gynecol Oncol 2008;108:577-83. [Crossref] [PubMed]
- Lin JY, DuBeshter B, Angel C, et al. Morbidity and recurrence with modifications of radical vulvectomy and groin dissection. Gynecol Oncol 1992;47:80-6. [Crossref] [PubMed]
- Gould N, Kamelle S, Tillmanns T, et al. Predictors of complications after inguinal lymphadenectomy. Gynecol Oncol 2001;82:329-32. [Crossref] [PubMed]
- Moore DH. Chemotherapy and radiation therapy in the treatment of squamous cell carcinoma of the vulva: Are two therapies better than one? Gynecol Oncol 2009;113:379-83. [Crossref] [PubMed]
- Crosbie EJ, Slade RJ, Ahmed AS. The management of vulval cancer. Cancer Treat Rev 2009;35:533-9. [Crossref] [PubMed]
- Wahlen SA, Slater JD, Wagner RJ, et al. Concurrent radiation therapy and chemotherapy in the treatment of primary squamous cell carcinoma of the vulva. Cancer 1995;75:2289-94. [Crossref] [PubMed]
- Landrum LM, Skaggs V, Gould N, et al. Comparison of outcome measures in patients with advanced squamous cell carcinoma of the vulva treated with surgery or primary chemoradiation. Gynecol Oncol 2008;108:584-90. [Crossref] [PubMed]
- Moore DH, Thomas GM, Montana GS, et al. Preoperative chemoradiation for advanced vulvar cancer: a phase II study of the Gynecologic Oncology Group. Int J Radiat Oncol Biol Phys 1998;42:79-85. [Crossref] [PubMed]
- Moore DH, Ali S, Koh WJ, et al. A phase II trial of radiation therapy and weekly cisplatin chemotherapy for the treatment of locally-advanced squamous cell carcinoma of the vulva: a gynecologic oncology group study. Gynecol Oncol 2012;124:529-33. [Crossref] [PubMed]
- Lee J, Kim SH, Kim G, et al. Treatment outcome in patients with vulvar cancer: comparison of concurrent radiotherapy to postoperative radiotherapy. Radiat Oncol J 2012;30:20-6. [Crossref] [PubMed]
- Zhou J, Shan G. The prognostic role of FIGO stage in patients with vulvar cancer: a systematic review and meta-analysis. Curr Med Res Opin 2016;32:1121-30. [Crossref] [PubMed]
- Tanaka Y, Ueda Y, Kakuda M, et al. Trends in incidence and long-term survival of Japanese women with vulvar cancer: a population-based analysis. Int J Clin Oncol 2019;24:1137-42. [Crossref] [PubMed]
- Te Grootenhuis NC, Pouwer AW, de Bock GH, et al. Prognostic factors for local recurrence of squamous cell carcinoma of the vulva: A systematic review. Gynecol Oncol 2018;148:622-31. [Crossref] [PubMed]
- Kunos C, Simpkins F, Gibbons H, et al. Radiation therapy compared with pelvic node resection for node-positive vulvar cancer: a randomized controlled trial. Obstet Gynecol 2009;114:537-46. [Crossref] [PubMed]
- Stecklein SR, Frumovitz M, Klopp AH, et al. Effectiveness of definitive radiotherapy for squamous cell carcinoma of the vulva with gross inguinal lymphadenopathy. Gynecol Oncol 2018;148:474-9. [Crossref] [PubMed]
- Natesan D, Susko M, Havrilesky L, et al. Definitive Chemoradiotherapy for Vulvar Cancer. Int J Gynecol Cancer 2016;26:1699-705. [Crossref] [PubMed]
- Rao YJ, Chin RI, Hui C, et al. Improved survival with definitive chemoradiation compared to definitive radiation alone in squamous cell carcinoma of the vulva: A review of the National Cancer Database. Gynecol Oncol 2017;146:572-9. [Crossref] [PubMed]
- Vorbeck CS, Jhingran A, Iyer RB, et al. Patterns of treatment failure in patients undergoing adjuvant or definitive radiotherapy for vulvar cancer. Int J Gynecol Cancer 2019; [Crossref]
- Kim Y, Kim JY, Kim JY, et al. Treatment outcomes of curative radiotherapy in patients with vulvar cancer: results of the retrospective KROG 1203 study. Radiat Oncol J 2015;33:198-206. [Crossref] [PubMed]
Cite this article as: Cheng HS, Wang L, Lu CH. Analyses of treatment outcomes in advanced vulvar cancer: treated with postoperative radiotherapy or definitive radiotherapy/chemoradiotherapy. Ther Radiol Oncol 2023;7:5.