
Radiotherapy to the rare penile metastases of rectal adenocarcinoma: a case report
Introduction
Malignant penile tumor is very rare, despite its nature is primary or secondary. Primary penile malignancy was reported with a very low incidence ranging from 0.58 to 1.3 cases per 100,000 people (1,2). As for secondary penile malignancy, the incidence is much rarer, with no more than 500 cases reported as yet. Since the scarcity of secondary penile malignancy, there is no standard treatment guideline for it. There were only sporadic cases showed palliative operation, chemotherapy, or radiotherapy as management. Surgery with penectomy remains the optimal choice with the longest median survival time of 1 year; radiotherapy has an average survival of 8 months (3). We would like to review a case with secondary penile malignancy who underwent regional radiotherapy to the penis. We presented this case in accordance with the CARE reporting checklist (available at https://tro.amegroups.com/article/view/10.21037/tro-22-26/rc).
Case presentation
A 69-year-old man visited our hospital in August 2018. He had suffered from melena for 6 months. After general survey, he was diagnosed with rectal cancer, adenocarcinoma, 10–15 cm from anal verge, cT3N1M0, stage 3B. Low anterior resection was performed on August 20th, 2018 and the pathology disclosed rectal adenocarcinoma, grade 3, pT4aN1b. Adjuvant concurrent chemoradiation was suggested consequently, but the patient received radiotherapy alone due to his refusal to chemotherapy. The radiation fields included primary rectal tumor surgical bed, involved lymph nodes surgical bed, and pelvic lymphatic drainage area. The total radiation dose of 5,400 cGy in 27 fractions was delivered by Linear accelerator with volumetric modulated arc therapy technique.
During regular follow-up, unfortunately, the image survey done a year later told the presentation of local recurrent rectal tumor, with liver and lung metastases. Recurrent rectal cancer, rcT4aN1nM1b was impressed. For salvage management, chemotherapy with FORFIRI (irinotecan + leucovorin + 5-fluorouracil) plus target therapy of Avastin were initiated in September 2019.
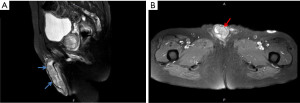
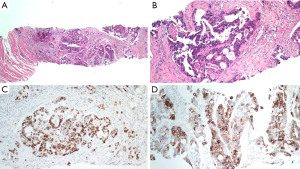
Besides, a bean-sized nodule at the left side of distal penis shaft was noticed, accompanied with mild tenderness. The nodule gradually enlarged in one month with painful sensation, dysuria, and aggravated urine frequency. Consequent magnetic resonance imaging (MRI) of the scrotum showed several well-defined penile tumors with hypointense signal, and heterogenous texture (Figure 1). Penile nodule biopsy reported with infiltrating carcinoma cells arranged in glandular and cribriform patterns in the desmoplastic stroma. Further immunohistochemical study demonstrated: Cytokeratin 20 (CK20)(+), Caudal-type homeobox 2 (CDX-2)(+) and Cytokeratin 7 (CK7)(+). The overall features are consistent with metastatic adenocarcinoma of colorectal origin (Figure 2).
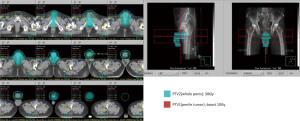
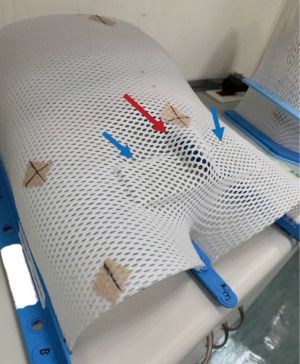
Even under salvage chemotherapy, the penile metastases were still progressing. For local control and symptom relief to the penile metastases, radiation oncology department was consulted. The Dosimetry and target delineation was planned as following. The gross tumor volume (GTV) circled the penile tumors, with an expansion of 0.3–0.5 cm to be the clinical target volume 1 (CTV1), and the planning target volume 1 (PTV1) was the same as CTV1. On the other hand, the clinical target volume 2 (CTV2) included the whole penile shaft, with an expansion of 0.5-1.0 cm into the planning target volume 2 (PTV2). Radiation dose of 50 Gy in 2 Gy daily fractions was prescribed to the PTV2 (the whole penis shaft), and the other 10 Gy in 2 Gy daily fractions was provided as a boost to PTV1 (the penile tumors). The course would be given 5 days per week by using 6 megavoltages (MV) photon beam under volumetric modulated arc therapy technique (Figure 3). Before each fraction treated, the patient would be placed in supine position, and stabilized in pelvis cast. Bilateral arms would be put on chest. There was a bridge made by Styrofoam and put across his thighs. His penis would be fixed on the Styrofoam bridge straightly, to avoid unnecessary skin folds formation (Figure 4).
The radiotherapy course was initiated in January 2020, but unexpectedly ended at the dose of 44 Gy on February 20th, 2020. Apparent skin reaction was observed since the 3rd week of irradiation. Grade III radiation dermatitis with an ulcer located at the ventral side of the penile root emerged. Thus, the irradiation plan was closed. Adequate wound care was given in ward during his admission for systemic therapy. The dermatitis and the ulcer all recovered well in a month. Meanwhile, chemotherapy and target therapy were maintained for his liver and lung metastases. But the patient finally expired on June 13th, 2020 due to respiratory failure and sepsis. The time interval from the diagnosis of his penile metastases to his death was approximately 6 months, and the interval from the end of radiotherapy was 4 months.
During follow-up in this time span presented above, the symptoms that he suffered from penile metastases, such as dysuria, painful nodule, and tenderness were relieved. Yet no newly growing penile lesions, urethral obstruction, or dermatitis were noticed. The irradiation played a palliative role in his penile metastases.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). We reviewed this case after 2 years of his death with no identifiable personal information of the patient in this article. Written informed consent was evitable under the permission of the Institutional Review Board (IRB) in Kaohsiung Medical University Hospital.
Discussion
The first description of secondary penile malignancy was published in 1870 by Eberth, with a case of penile metastases from rectal adenocarcinoma (4). In 1872, Roberts reported the first secondary penile tumor from the genitourinary origin (5,6). Among all the penile metastases reported before, one-third of the penile metastases were diagnosed in synchrony with the primary tumors, while the remaining two-thirds were noted within 18 months after the primary ones were diagnosed (7). When penile metastases were diagnosed, the majority of cases (90%) were found to have disseminated disease, with a survival time no longer than 12 months, which was associated with a poor prognosis (5,8).
The primary site of penile metastases generally comes from pelvic region, especially the bladders (30–35%) and prostates (28–30%) (9,10). Other cases were from the rectosigmoid colons (13%), kidneys (8–10%), and testes (5%) (9,10). Further lesser primary sites included the gastrointestinal or respiratory tract origins (11). Mostly, it goes though retrograde venous route (12,13).
Penile metastases from rectal adenocarcinoma are relatively rare, and there were some theories about how it goes: arterial or venous spread, retrograde lymphatic spread, or direct metastatic infiltration. But the exact metastatic route was still pending proven. The most common symptoms of penile tumor presented with a nodular mass, malignant priapism, pain sensation, tenderness, and difficult urination with urine retention (14,15).
Since the scarcity of penile metastases, there was no standard treatment guideline published. Only sporadic cases reported the use of palliative operation or chemotherapy. As for palliative radiotherapy, dose of 30 Gy in 3 Gy daily fraction had been mentioned, but further experience was hardly possible to find (16). On the contrary, for primary penile cancer, partial operation, radical operation, or chemotherapy, are proof options according to the National Comprehensive Cancer Network (NCCN).
It showed that the managements of penile metastases were most in palliative intent and not standardized, due to the limited survival time (10). After treatment to the penile metastases, such as local tumor excision, chemotherapy, or radiotherapy alone, the reported survival time of such cases usually ranged from 7 months to 2 years. Long-term survivals from 21 months to 9 years might be seen after aggressive surgical treatment such as penile amputation (5).
We presented this case with three focuses. First, the scarcity of metastatic penile tumor; second, the rare origin beside the other common primary malignancy; third, the occurrence of penile metastases indicates a very poor prognosis. For such patient, applying regional radiotherapy to the penis could play a palliative role for symptom relief, and might remain a better quality of life for the rest of the patient’s life.
Acknowledgments
We thank all the members of the Department of Radiation Oncology of Kaohsiung Medical University Hospital for their support.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tro.amegroups.com/article/view/10.21037/tro-22-26/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tro.amegroups.com/article/view/10.21037/tro-22-26/coif). MYH serves as an unpaid editorial board member of Therapeutic Radiology and Oncology from May 2022 to April 2024. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baldur-Felskov B, Hannibal CG, Munk C, et al. Increased incidence of penile cancer and high-grade penile intraepithelial neoplasia in Denmark 1978-2008: a nationwide population-based study. Cancer Causes Control 2012;23:273-80. [Crossref] [PubMed]
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol 2007;25:361-7. Erratum in: Urol Oncol 2008;26:112. [Crossref] [PubMed]
- Kragelj B. Irradiation of regionally advanced carcinoma of the penis. Radiol Oncol 2009;43:41-6. [Crossref]
- Zhang K, Da J, Yao HJ, et al. Metastatic tumors of the penis: a report of 8 cases and review of the literature. Medicine (Baltimore) 2015;94:e132. [Crossref] [PubMed]
- Chaux A, Amin M, Cubilla AL, et al. Metastatic tumors to the penis: a report of 17 cases and review of the literature. Int J Surg Pathol 2011;19:597-606. [Crossref] [PubMed]
- Abeshouse BS, Abeshouse GA. Metastatic tumors of the penis: a review of the literature and a report of two cases. J Urol 1961;86:99-112. [Crossref] [PubMed]
- Kimura Y, Shida D, Nasu K, et al. Metachronous penile metastasis from rectal cancer after total pelvic exenteration. World J Gastroenterol 2012;18:5476-8. [Crossref] [PubMed]
- Park JC, Lee WH, Kang MK, et al. Priapism secondary to penile metastasis of rectal cancer. World J Gastroenterol 2009;15:4209-11. [Crossref] [PubMed]
- Zhu YP, Yao XD, Zhang HL, et al. Penile metastasis from primary bladder cancer: a study of 8 cases and review of the literature. Onkologie 2012;35:196-9. [Crossref] [PubMed]
- Cherian J, Rajan S, Thwaini A, et al. Secondary penile tumours revisited. Int Semin Surg Oncol 2006;3:33. [Crossref] [PubMed]
- Karanikas C, Ptohis N, Mainta E, et al. Pulmonary adenocarcinoma presenting with penile metastasis: 204 a case report. J Med Case Reports 2012;6:252. [Crossref] [PubMed]
- PAQUIN AJ Jr. ROLAND SI. Secondary carcinoma of the penis; a review of the literature and a report of nine new cases. Cancer 1956;9:626-32. [Crossref] [PubMed]
- Bell S, Sasaki J, Sinclair G, et al. Understanding the anatomy of lymphatic drainage and the use of blue-dye mapping to determine the extent of lymphadenectomy in rectal cancer surgery: unresolved issues. Colorectal Dis 2009;11:443-9. [Crossref] [PubMed]
- Efared B, Ebang GA, Tahirou S, et al. Penile metastasis from rectal adenocarcinoma: a case report. BMC Res Notes 2017;10:564. [Crossref] [PubMed]
- Haddad FS, Manne RK. Involvement of the penis by rectocolic adenocarcinoma. Report of a case and review of the literature. Dis Colon Rectum 1987;30:123-9. [Crossref] [PubMed]
- Tello TL, Zeidan YH, Bush K, et al. Penile metastases originating from a pancreatic primary tumor: a case report. J Radiat Oncol 2013;2:107-12. [Crossref]
Cite this article as: Weng YY, Yang SF, Huang MY. Radiotherapy to the rare penile metastases of rectal adenocarcinoma: a case report. Ther Radiol Oncol 2023;7:12.


