
Hemangiopericytoma in the central nervous system: a case report
Introduction
Hemangiopericytomas (HPCs) are rare soft tissue neoplasms. They are sarcomatous lesions arising from the smooth muscle around vessels. HPCs are most common in the head and neck region, while they are less commonly seen in the central nervous system (CNS). Here, we report a patient of CNS HPC, who experienced long-term follow-up after the treatment of surgery and radiotherapy, and also reviewed the literature on this rare tumor. We present this case in accordance with the CARE reporting checklist (available at https://tro.amegroups.com/article/view/10.21037/tro-22-21/rc).
Case presentation
A 36-year-old man with no significant past medical history presented to the outpatient department in November 2009 with fever up to 40 degrees Celsius and headache over the posterior head region for 2 days. With the suspicion of meningitis, a brain magnetic resonance imaging (MRI) was done which showed a 57 mm × 40 mm × 70 mm lobulated extra-axial tumor over the right cerebellar tentorium with focal mass effect and resultant mild secondary non-communicating obstructive hydrocephalus (Figure 1). The possibility of extra-axial tumor such as pedunculated meningioma was considered. He underwent an excision of the lobulated mass which was sent for histopathological diagnosis. This revealed randomly oriented monomorphous tumor cells with little intervening fibrosis. The cytoplasm was scant and cell borders were indistinct. The nuclei were round to oval with moderately dense chromatin, inconspicuous nucleoli to focal areas of vesicular nuclei and mild to moderate atypia. The mitotic activity was mildly increased. No necrosis was seen in the sections. Focal mild hemorrhage and embolization material in vessels were noted. The immunohistochemical study demonstrated positive for CD34, CD99, and reticulin along with an increased Ki-67 proliferative index suggestive of a HPC.
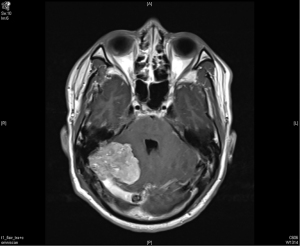
The patient recovered well after the surgery. However, a follow up image in March 2010 revealed a focal small recurrent tumor over the original surgical tumor bed (Figure 2). Due to the recurrence, patient underwent radiotherapy as stereostatic radiosurgery (SRS) with a dose of 14 Gy in one fraction in May 2010, achieving a good response. He was tumor free for the next 5 years.
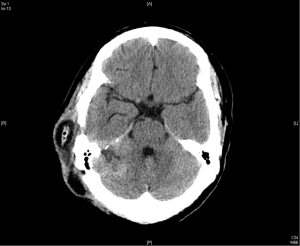
In April 2015, the patient took another course of radiotherapy as fractionated schedule of 5,940 cGy in 33 fractions, with 180 cGy in one fraction per day, 5 days a week, due to recurrence again nearby the previous tumor bed (Figure 3). The treatment volume of radiotherapy included the gross recurrent tumor with perifocal edematous change. This time, the recurrent tumor was under control and stable for about one and half year (Figure 4).
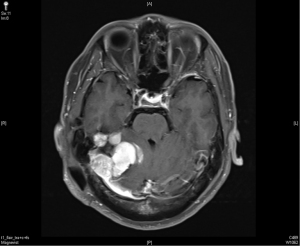
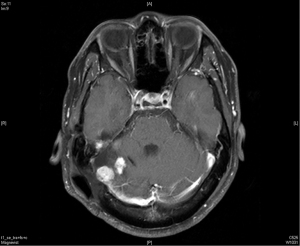
In December 2016, his brain MRI showed size increase of the previous recurrent tumor, and the patient received the third course of radiotherapy with a cumulative dose as 6,000 cGy in 30 fractions. No more enlargement of tumor was noted in the next 3 years.
Dosage distribution area of the 1st RT course (SRS) was based on the small focal recurrent tumor over the original surgical tumor bed in 2010. Five years later in 2015, the 2nd RT course (5,940 cGy in 33 fractions) was given. Six years later in 2016, the 3rd RT course (6,000 cGy in 30 fractions) was proceeded. According to the serials of the following images studies, there seemed no radiation change within the RT field, and also the patient did not present any obvious CNS dysfunction related to irradiation effects.
Until in December 2019, much progression of the aforementioned recurrent tumor was observed as increased size of prior lesions and increased number of enhancing nodules at the previous tumor areas with diffuse meningeal infiltration (Figure 5). Neither additional radiotherapy nor further surgery was feasible with the concern of possible irreversible brain function impairment. The patient decided to take medication treatment as pain killers and others for symptoms relief. He remained regular follow-up in the outpatient department. Table 1 showed the summary from March 2010 until December 2019 of the sites of the recurrence, clinical features, dosage of radiotherapy, and duration of tumor free after radiotherapy.
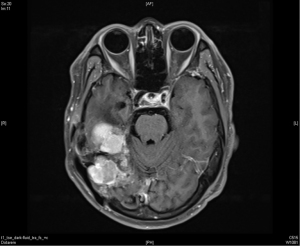
Table 1
| Sites of the recurrence | Clinical features | Dosage of radiotherapy | Duration of tumor free after radiotherapy | |
|---|---|---|---|---|
| March 2010 | A focal small recurrent tumor over the original surgical tumor bed over right cerebellar tentorium | Headache | Stereotactic radiosurgery with a dose of 14 Gy in one fraction | Five years |
| April 2015 | Recurrent lobulated extra-axial pedunculated tumors over right cerebellar tentorium | Unsteady gait, vertigo |
5,940 cGy in 33 fractions | One year and 8 months |
| December 2016 | Enlargement of supratentorial portion of tumor over right cerebellar tentorium | Headache | 6,000 cGy in 30 fractions | Three years |
| December 2019 | Increased size and increased number of enhancing nodules over the right temporal and cerebellar regions | Ataxia, headache, dizziness | Additional radiotherapy was not feasible | – |
All procedures performed in this study were in accordance with the ethical standards of the research ethics committee of our institute (Institutional Review Board, Changhua Christian Hospital, Taiwan; IRB number: 200835) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
HPC is an uncommon neoplasm arising from the Zimmerman pericytes around capillaries and postcapillary venules. It was first recognized by Stout and Murray in 1942 (1). Most HPCs are located in the musculoskeletal system and the skin. CNS HPCs are rare, and the prevalence rate is around only 1% (2). In CNS, HPC usually arises from the pericytes of the meningeal capillaries and presents as a solid mass attached to the meninges, resembling a meningioma. HPCs of CNS are further categorized into low-grade (WHO grade I and II) or high-grade (WHO grade III, anaplastic) neoplasms, of which the latter is more aggressive (2).
HPCs usually affect adults in their fourth to fifth decade of life, with a mean age ranging from 38 to 44.9 years and a slight male predominance (3), which is unlike meningiomas. The clinical symptoms generally consist of headache and dizziness, as well as damage to the corresponding functional areas where the tumor is located, such as blurred vision and limb movement disorders related to tumor sites and locations.
Meningeal solitary fibrous tumors (SFTs) and HPCs were thought to represent distinct entities, the identification of NAB2–STAT6 fusion as a defining molecular alteration in both tumors has led to a combined classification of these tumors at both dural and extradural sites (4). Before 2016, meningeal SFTs/HPCs had been categorized into two groups—classic, typically more benign SFTs, and HPCs, which are usually more aggressive tumors. Nuclear STAT6 signals in immunohistochemistry are reported to be useful for distinguishing HPC/SFT from other mesenchymal neoplasms (5).
CNS HPC mimics meningioma and may be confused with it, but it is more aggressive. It was ever divided into a subtype of meningioma for a long time. The difference between HPC and meningioma in CT scan or MRI may be helpful in the differential diagnosis of them, but even though, these tumors still mimic meningioma on imaging, and thus the histopathological diagnosis holds the key; that means the definitive diagnosis of HPC mainly depends on unique cell morphology and results of immunohistochemistry stains in pathology (6).
Meningeal HPCs have a local recurrence rate as high as 80%, and late metastases can occur in 50–80% of patients. The mean period for local recurrence is 75 months, while metastases arising 10 years post diagnosis of the primary tumor are not uncommon (7). The nature of the neoplasm reflects the diversity of tumor behavior. The prognosis is determined by the site, volume of the neoplasm and whether there is metastasis. It was found that the tentorial and posterior fossa tumors are more aggressive than the supratentorial tumors (8). In addition to these factors, the treatment modality also affects the prognosis of CNS HPCs.
Complete surgical excision is the mainstay of the treatment in CNS HPCs. Patients undergoing gross total surgical resection of the tumor as the initial surgery achieve good long-term local control (9). The average recurrence-free survival of patients with complete resection was 105.7 months, and that of patients with partial resection was 24.6 months (3). Moreover, a lot of studies revealed that post-operative adjuvant radiotherapy is indispensable in the treatment of CNS HPCs. A higher 5-year recurrence-free survival (57%) was noted in patients with adjuvant radiotherapy after surgery compared to 28% with gross total resection alone (10). Patients receiving adjuvant radiotherapy tend to survive longer without recurrence (3) (mean period of 85.4 months versus 10.7 months). The overall survival was significantly better in patients with adjuvant radiotherapy than without (11) (10-year overall survival as 66.2% versus 40.7%). Remarkably, if patients are given adjuvant radiotherapy after subtotal surgical resection, they will experience a non-inferior overall survival in comparison with gross total resection alone (3) (5-year overall survival as 89% versus 89.2%). In contrast to adjuvant radiotherapy, chemotherapy only provides negligible benefits. There is no definitive beneficial evidence for adjuvant chemotherapy (7).
Late extracranial metastases of CNS HPCs have been described at an average interval of 113 months (12-14). Bone, lung, and liver are the most common sites of metastasis (3,12,15). Surgical resection or local radiotherapy may be advocated in the treatment of distant metastasis (12).
Our patient survived for more than 10 years, which is compatible with the literature review. However, local recurrences could not be avoided, and eventually no further treatment was feasible to manage his disease progression as he had received radical surgery and high-dose radiotherapy for three courses. Regular follow-up with medications for symptomatic relief was the only treatment that could be provided.
In conclusion, HPC in CNS is a rare neoplasm, which with the appropriate treatment modalities (gross total resection and adjuvant radiotherapy) confers a long-term survival, but local recurrence or distant metastasis may occur throughout the follow-up duration.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tro.amegroups.com/article/view/10.21037/tro-22-21/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tro.amegroups.com/article/view/10.21037/tro-22-21/coif). JCL serves as an unpaid editorial board member of Therapeutic Radiology and Oncology from May 2022 to April 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the research ethics committee of our institute (Institutional Review Board, Changhua Christian Hospital, Taiwan; IRB number: 200835) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stout AP, Murray MR. Hemangiopericytoma: a vascular tumor featuring Zimmermann’s pericytes. Ann Surg 1942;116:26-33. [Crossref] [PubMed]
- Park BJ, Kim YI, Hong YK, et al. Clinical analysis of intracranial hemangiopericytoma. J Korean Neurosurg Soc 2013;54:309-16. [Crossref] [PubMed]
- Liu F, Cai B, Du Y, et al. Diagnosis and treatment of hemangiopericytoma in the central nervous system. J Cancer Res Ther 2018;14:1578-82. [Crossref] [PubMed]
- Giordan E, Marton E, Wennberg AM, et al. A review of solitary fibrous tumor/hemangiopericytoma tumor and a comparison of risk factors for recurrence, metastases, and death among patients with spinal and intracranial tumors. Neurosurg Rev 2021;44:1299-312. [Crossref] [PubMed]
- Macagno N, Figarella-Branger D, Mokthari K, et al. Differential Diagnosis of Meningeal SFT-HPC and Meningioma: Which Immunohistochemical Markers Should Be Used? Am J Surg Pathol 2016;40:270-8. [Crossref] [PubMed]
- Natteru P, Ramachandran Nair L, Luzardo G, et al. Meningeal Hemangiopericytoma Presenting as Pure Gerstmann Syndrome: A Double Rarity. Cureus 2021;13:e15863. [Crossref] [PubMed]
- Halperin EC, Wazer DE, Perez CA, et al. Unusual Nonepithelial Tumors of the Head and Neck. In: Perez & Brady's Principles and Practice of Radiation Oncology, 7th ed. Philadelphia: Lippincott Williams and Wilkins; 2018.
- Singh SB, Vanathi N, Shah VM. A rare case of CNS hemangiopericytoma presenting with papilledema. GMS Ophthalmol Cases 2019;9:Doc32. [Crossref] [PubMed]
- Wu W, Shi JX, Cheng HL, et al. Hemangiopericytomas in the central nervous system. J Clin Neurosci 2009;16:519-23. [Crossref] [PubMed]
- Staples JJ, Robinson RA, Wen BC, et al. Hemangiopericytoma--the role of radiotherapy. Int J Radiat Oncol Biol Phys 1990;19:445-51. [Crossref] [PubMed]
- Redmond KJ, Gullett NP, Kleinberg L, et al. Hemangiopericytoma of the Central Nervous System: Analysis of Current National Patterns of Care. Proceedings of the 52nd Annual ASTRO Meeting, 2010.
- Lo RC, Suriawinata AA, Rubin BP. Liver metastasis of meningeal hemangiopericytoma: a study of 5 cases. Clin Mol Hepatol 2016;22:188-91. [Crossref] [PubMed]
- Schiariti M, Goetz P, El-Maghraby H, et al. Hemangiopericytoma: long-term outcome revisited. Clinical article. J Neurosurg 2011;114:747-55. [Crossref] [PubMed]
- Kim JH, Jung HW, Kim YS, et al. Meningeal hemangiopericytomas: long-term outcome and biological behavior. Surg Neurol 2003;59:47-53; discussion 53-4. [Crossref] [PubMed]
- Satayasoontorn K, Righi A, Gambarotti M, et al. Meningeal hemangiopericytoma only diagnosed at the time of late bone metastasis. Skeletal Radiol 2014;43:1543-9. [Crossref] [PubMed]
Cite this article as: Huang CC, Chang TH, Lin JC. Hemangiopericytoma in the central nervous system: a case report. Ther Radiol Oncol 2023;7:8.


