
Contralateral neck nodal outcomes and recurrence pattern of small well-lateralized oral cavity carcinoma—a single institution experience
Introduction
Elective neck dissection on the contralateral side of well-lateralized oral-cavity cancers may not be performed during surgery, depending on the clinical tumor size or nodal status. Under some circumstances, post-operative radiotherapy (RT) is administered for pathologically proven high-risk head and neck malignancies, which are associated with increased local and regional nodal control (1). In past decades, the convention was to irradiate bilateral neck region lymph nodes once RT was administered; however, if contralateral nodal negativity is indicated by pathology, the necessity of contralateral neck irradiation remains controversial. Moreover, RT to the bilateral neck area is associated with acute and long-term adverse effects, reducing patient quality of life.
Previous studies revealed relatively low contralateral nodal recurrence rates of well-lateralized head and neck cancers (2-4). However, most of this research was based on oropharyngeal squamous cell carcinoma, and only a few cases of oral-cavity cancers have been reported (5,6). Whether the results of oropharyngeal cancer can be extrapolated to oral-cavity cancer remains a controversial question.
Thus, we conducted a retrospective study, analyzing small, well-lateralized oral-cavity cancers treated primarily with surgical intervention, to evaluate outcomes and patterns of contralateral nodal failure with/without contralateral neck irradiation.
Methods
Patients and staging workup
Between 2007 and 2017, patients with primary tumors of non-metastatic, well-lateralized oral-cavity cancers, mainly in the buccal, gingival, and retromolar regions, were identified. Well-lateralized was defined as tumor located more than 1 cm from midline of maxilla, mandible or hard palate. At a minimum, the pre-treatment staging workups included chest X-rays, contrast or non-contrast computed tomography (CT) or magnetic resonance imaging (MRI), and abdominal ultrasounds. If necessary, bone scans and/or positron emission tomography/CT (PET/CT) were used to rule out possible distant metastases. Patients whose pathology indicated pT1/2 or pN0-2b stage cancer according to the American Joint Committee on Cancer (AJCC) Staging Manual 6th/7th edition (7,8) were included in this study. Those simultaneously diagnosed with second primary cancers, with histories of cancer, or non-squamous cell histology oral-cavity cancer were excluded from the analysis.
Treatment
All patients underwent curative surgical excision as their first treatment, including composite tumor resection and/or neck dissection. Performance of ipsilateral or bilateral neck dissection was determined according to pre-operative CT or MRI and at the surgeon’s discretion. Patients with pathological risk factors for possible recurrence, such as positive margins, involved nodes, or extranodal extension, received post-operative RT/chemotherapy [following National Comprehensive Cancer Network (NCCN) guidelines].
Adjuvant RT was administered by either intensity-modulated radiotherapy (IMRT) or volumetric-modulated arc therapy (VMAT) using a megavoltage linear accelerator. Planning CT was obtained after the patient was immobilized with a thermoplastic mask. The following radiation doses were administered: 45–54 Gy to neck regional lymph nodes and 59.4–72 Gy to surgical tumor beds and high-risk nodal areas, which were all in 1.8–2 Gy per fraction. Administration of ipsilateral or bilateral neck irradiation was at the radiation oncologist’s discretion according to the pathology report, such as tumor stage, nodal status, or extranodal extension status. Concurrent chemotherapy was administered at the medical oncologist’s discretion.
Follow-ups
Regular follow-ups were arranged every 2–3 months in the first two years and then every 4–6 months afterwards. On each visit, oral inspection with neck palpation and the occasional endoscopic examination were done regularly. Imaging studies (CT or MRI) were done every 3–6 months during the follow-up period. Tissue pathology proof would be obtained if clinically or radiologically recurrence were suspected.
Statistical analysis
The primary end point of this study was contralateral nodal failure-free survival (cNFFS). The crude rate of contralateral neck failure was also demonstrated. Secondary end points included overall survival (OS) and event-free survival (EFS).
Failure on the ipsilateral or contralateral side was well-documented, so cNFFS could be defined as the amount of time between the day of operation and the day of contralateral nodal failure at the first failure site. OS was defined as the amount of time between the day of operation and the day of death from any cause or the last follow-up. EFS was defined as the amount of time between the day of operation and the day of local recurrence, nodal failure, or distant metastasis, whichever came first. For patients who received adjuvant RT, we conducted subgroup analyses of cNFFS, OS, and EFS.
The Kaplan-Meier method was used to estimate all the survival endpoints. The survival between treatment groups was compared with a stratified log-rank test. All statistical analysis was performed using Statistical Product and Service Solutions (SPSS) software, version 22.0 (SPSS Inc., Chicago, IL, USA) and R software (version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria). Two-tailed P<0.05 was considered statistically significant.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of National Cheng Kung University Hospital (IRB number: A-ER-111-181). The requirement for informed consent from the study subjects was waived due to the retrospective study design.
Results
Study population
From May 2007 to December 2017, 177 patients in our institution were analyzed. The median follow-up was 79.7 months (range, 2.2–178.1 months). The median age at diagnosis was 54 years (range, 31–91 years).
Patient and tumor characteristics are listed in Table 1. The main tumor sites included buccal (139, 78.5%), gingival (31, 17.5%), and retromolar (7, 4.0%) subsites. The pathological stages were II (115, 65%), III (22, 12.4%), and IVA (40, 22.6%) with tumor stages of T1 (18, 10.1%) and T2 (159, 89.9%) and nodal stages of N0 (115, 65%), N1 (22, 12.4%), and N2 (40, 22.6%). One hundred forty-five (81.9%) patients received ipsilateral neck dissections, 20 (11.3%) patients received adjuvant RT alone, and 36 (20.3%) patients received concurrent chemoradiotherapy (CCRT).
Table 1
| Characteristics | OP | OP + RT | P value |
|---|---|---|---|
| Patient number | 121 | 56 | |
| Median age in years [range] | 53 [31–86] | 50 [32–91] | 0.272 |
| Gender, n (%) | 0.578 | ||
| Male | 110 (90.9) | 53 (94.6) | |
| Female | 11 (9.1) | 3 (5.4) | |
| Tumor site, n (%) | 0.111 | ||
| Buccal | 91 (75.2) | 48 (85.7) | |
| Gingival | 26 (21.5) | 5 (8.9) | |
| Retromolar | 4 (3.3) | 3 (5.4) | |
| Tumor stage, n (%) | <0.001 | ||
| 1 | 5 (4.1) | 13 (23.2) | |
| 2 | 116 (95.9) | 43 (76.8) | |
| Nodal stage, n (%) | <0.001 | ||
| 0 | 104 (86.0) | 11 (19.6) | |
| 1 | 12 (9.9) | 10 (17.9) | |
| 2 | 5 (4.1) | 35 (62.5) | |
| Stage, n (%) | <0.001 | ||
| II | 104 (86.0) | 11 (19.6) | |
| III | 12 (9.9) | 10 (17.9) | |
| IVA | 5 (4.1) | 35 (62.5) | |
| Neck dissection side, n (%) | 0.009 | ||
| Ipsilateral | 98 (81.0) | 47 (83.9) | |
| Bilateral | 9 (7.4) | 9 (16.1) | |
| No neck dissection | 14 (11.6) | 0 (0.0) | |
| Tumor differentiation, n (%) | 0.001 | ||
| Well | 68 (56.2) | 15 (26.8) | |
| Moderate | 48 (39.7) | 35 (62.5) | |
| Poor | 4 (3.3) | 6 (10.7) | |
| Data missing | 1 (0.8) | 0 (0.0) | |
| Tumor size, n (%) | 0.221 | ||
| ≤2 cm | 14 (11.6) | 12 (21.4) | |
| >2 cm, ≤4 cm | 103 (85.1) | 42 (75.0) | |
| >4 cm | 3 (2.5) | 2 (3.6) | |
| Data missing | 1 (0.8) | 0 (0.0) | |
| Perineural invasion, n (%) | <0.001 | ||
| Yes | 20 (16.5) | 28 (50.0) | |
| No | 98 (81.0) | 28 (50.0) | |
| Data missing | 3 (2.5) | 0 (0.0) | |
| Lymphovascular invasion, n (%) | <0.001 | ||
| Yes | 12 (9.9) | 25 (44.6) | |
| No | 106 (87.6) | 31 (55.4) | |
| Data missing | 3 (2.5) | 0 (0.0) | |
| Extracapsular spread, n (%) | <0.001 | ||
| Yes | 4 (3.3) | 20 (35.7) | |
| No | 114 (94.2) | 36 (64.3) | |
| Data missing | 3 (2.5) | 0 (0.0) | |
| Margin status, n (%) | 0.013 | ||
| ≤1 mm or involved (inadequate) | 2 (1.7) | 7 (12.5) | |
| 1–3 mm (close) | 53 (43.8) | 26 (46.4) | |
| >3 mm (free) | 65 (53.7) | 22 (39.3) | |
| Data missing | 1 (0.8) | 1 (1.8) | |
| Adjuvant chemotherapy, n (%) | <0.001 | ||
| No | 119 (98.3) | 20 (35.7) | |
| Yes | 2 (1.7) | 36 (64.3) | |
OP, operation; RT, radiotherapy.
Patient and tumor characteristics of those received ipsilateral and bilateral neck RT are listed in Table 2. Thirty-one patients received ipsilateral neck nodal RT, while 25 patients received bilateral neck nodal irradiation. All characteristics showed no statistical difference between two subgroups except pathological and nodal stage (P=0.015), and neck dissection side (P=0.011).
Table 2
| Characteristics | Ipsilateral RT | Bilateral RT | P value |
|---|---|---|---|
| Patient number | 31 | 25 | |
| Median age in years [range] | 50 [37–91] | 49 [32–65] | 0.499 |
| Gender, n (%) | 0.316 | ||
| Male | 28 (90.3) | 25 (100.0) | |
| Female | 3 (9.7) | 0 (0.0) | |
| Tumor site, n (%) | 0.538 | ||
| Buccal | 28 (90.3) | 20 (80.0) | |
| Gingival | 2 (6.5) | 3 (12.0) | |
| Retromolar | 1 (3.2) | 2 (8.0) | |
| Tumor stage, n (%) | 0.657 | ||
| 1 | 6 (19.4) | 7 (28.0) | |
| 2 | 25 (80.6) | 18 (72.0) | |
| Nodal stage, n (%) | 0.015 | ||
| 0 | 10 (32.3) | 1 (4.0) | |
| 1 | 3 (9.7) | 7 (28.0) | |
| 2 | 18 (58.1) | 17 (68.0) | |
| Stage, n (%) | 0.015 | ||
| II | 10 (32.3) | 1 (4.0) | |
| III | 3 (9.7) | 7 (28.0) | |
| IVA | 18 (58.1) | 17 (68.0) | |
| Neck dissection side, n (%) | 0.011 | ||
| Ipsilateral | 30 (96.8) | 17 (68.0) | |
| Bilateral | 1 (3.2) | 8 (32.0) | |
| Tumor differentiation, n (%) | 0.359 | ||
| Well | 6 (19.4) | 9 (36.0) | |
| Moderate | 21 (67.7) | 14 (56.0) | |
| Poor | 4 (12.9) | 2 (8.0) | |
| Tumor size, n (%) | 0.897 | ||
| ≤2 cm | 6 (19.4) | 6 (24.0) | |
| >2 cm, ≤4 cm | 24 (77.4) | 18 (72.0) | |
| >4 cm | 1 (3.2) | 1 (4.0) | |
| Perineural invasion, n (%) | 1 | ||
| Yes | 16 (51.6) | 12 (48.0) | |
| No | 15 (48.4) | 13 (52.0) | |
| Lymphovascular invasion, n (%) | 0.206 | ||
| Yes | 11 (35.5) | 14 (56.0) | |
| No | 20 (64.5) | 11 (44.0) | |
| Extracapsular spread, n (%) | 0.378 | ||
| Yes | 9 (29.0) | 11 (44.0) | |
| No | 22 (71.0) | 14 (56.0) | |
| Margin status, n (%) | 0.23 | ||
| ≤1 mm or involved (inadequate) | 6 (19.4) | 1 (4.0) | |
| 1–3 mm (close) | 14 (45.2) | 12 (48.0) | |
| >3 mm (free) | 10 (32.3) | 12 (48.0) | |
| Data missing | 1 (3.2) | 0 (0.0) | |
| Adjuvant chemotherapy, n (%) | 0.054 | ||
| No | 15 (48.4) | 5 (20.0) | |
| Yes | 16 (51.6) | 20 (80.0) | |
RT, radiotherapy.
Outcomes
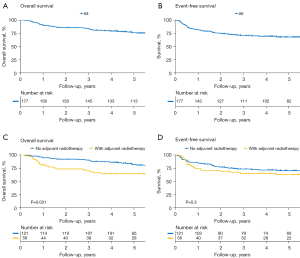
The primary endpoint, 5-year cNFFS, was 97.4% (Figure 1A), and the crude rate of contralateral neck failure was 3.4%. Patients received post-operative RT or not showed no statistical significance in cNFFS (Figure 1B). The 5-year OS and EFS were 75.7% and 67.9%, respectively (Figure 2A,2B); the survival difference between patients received post-operative RT or not were also shown in Figure 2C,2D.
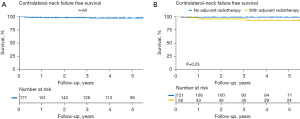
Regional neck recurrence was observed in 20 patients (11.3%); recurrences were in the ipsilateral, contralateral, and bilateral neck for 14, 4, and 2 patients, respectively. Of the 6 patients with contralateral/bilateral regional neck recurrence, 4 had local recurrence prior to regional recurrence, 1 experienced simultaneous local and regional neck recurrence, and 1 had regional neck recurrence prior to local recurrence.
Subgroup analysis
Of the 56 patients who had received RT, there was no statistically significant difference in cNFFS (92.6% vs. 93.3%; P=0.703), 5-year OS rate (67.2% vs. 60.0%; P=0.259), and EFS rate (69.5% vs. 54.0%; P=0.210) in patients receiving ipsilateral and bilateral neck irradiation (Figure 3).
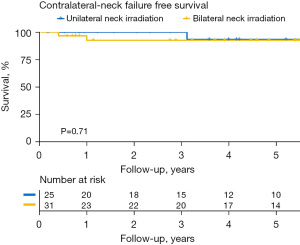
In patients who had received RT, regional neck recurrence was noted in 4 patients (7.1%); the recurrences were in the ipsilateral, contralateral, and bilateral neck regions in 1, 2, and 1 patients, respectively. Of the 3 patients who suffered from contralateral/bilateral neck recurrence, 2 had local recurrence prior to neck recurrence, and 1 had local recurrence after neck recurrence during follow-up. One of the 3 patients who experienced contralateral neck nodal recurrence received post-operative bilateral neck irradiation.
Discussion
This retrospective cohort study indicated a 5-year cNFFS of 97.4%, with a crude rate of contralateral neck recurrence of 3.4% in the patients with small, well-lateralized oral-cavity cancers. Previous retrospective studies had shown relatively low contralateral recurrence rates in lateralized head and neck patients of 5% (2), 6.1% (3), and 3% (9), which supports our study results. However, these studies included various subsites for head and neck cancer. This heterogeneity might have impacted the determined contralateral recurrence rates.
In addition, a retrospective study reported that midline involvement in oropharyngeal carcinomas was a significant prognostic factor in contralateral regional failure (5). Therefore, we chose to focus on patients with relatively lateralized tumors without midline involvement, such as buccal, gingival, and retromolar tumors.
To date, there is still no compelling evidence indicating whether contralateral neck dissection or irradiation should be administered in patients who are negative in the contralateral neck. In conventional clinical practice, once adjuvant RT is administered, it is unclear whether contralateral irradiation should be administered to these patients, especially in those with adverse pathological features [e.g., extracapsular spread (ECS)-positive or inadequate surgical margin status]. Our study results suggest that neither pathological factors nor ipsilateral/contralateral neck irradiation was significantly associated with cNFFS. However, we could not address the true impacts of contralateral neck radiation on outcomes because there were few events of contralateral neck recurrence in our subgroup analysis. The opportunity to omit unnecessary treatments without compromising cure rates prompts further investigation.
The pathological features, neck radiation sites, and relationships between local and contralateral neck failures for the 6 patients who experienced contralateral neck failure in our study are summarized in Table 3.
Table 3
| No. | Age (years old) | T stage | N stage | Tumor site | Margin (mm) | PNI | LVI | ECS | RT site | Chemotherapy | cNF time (years) | Local failure and relation to cNF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | 2 | 1 | Retromolar | 10 | + | − | − | – | – | 6.3 | Prior to cNF |
| 2 | 44 | 2 | 2b | Buccal | 10 | + | + | + | Ipsilateral | Cisplatin | 0.4 | cNF occurred first |
| 3 | 53 | 2 | 0 | Gingival | 6 | − | − | − | – | – | 5.6 | Prior to cNF |
| 4 | 64 | 2 | 0 | Buccal | 2 | + | − | − | Ipsilateral | – | 1.0 | Prior to cNF |
| 5 | 41 | 2 | 1 | Buccal | 5 | − | − | − | – | – | 0.2 | At the same time |
| 6 | 56 | 2 | 1 | Retromolar | 10 | − | − | − | Bilateral | – | 3.1 | Prior to cNF |
PNI, perineural invasion; LVI, lymphovascular invasion; ECS, extra-capsular spread; RT, radiotherapy; cNF, contralateral nodal failure.
All these 6 patients underwent ipsilateral neck dissection during surgery. All were classified as pathological stage T2, and 4 had positive neck lymph node metastases. There was no obvious correlation between the characteristics mentioned above and contralateral neck nodal recurrence after analysis. Only 1 had characteristics of ECS, which is currently regarded as a mainly adverse feature of prognosis. Another patient developed contralateral neck nodal recurrence even after receiving bilateral neck irradiation, which could be due to the relatively low treatment dose (45 Gy). It was noteworthy that 5 of 6 (83.3%) contralateral neck nodal recurrences occurred after, or simultaneously with, local failure. Only 1 patient suffered from isolated contralateral neck nodal recurrence prior to local failure. Consequently, better local control might result in lower contralateral nodal recurrence rates, and more intense follow-ups or image studies should be considered once local recurrence has occurred.
The study is limited because it is a single-institution, non-randomized, and retrospectively reviewed cohort study. The small sample size and low primary endpoint events during follow-up could contribute to inconclusive results and insufficient study power to detect risk factors for contralateral neck recurrence. Therefore, extrapolation of the results from a wider, well-lateralized oral-cavity cancer population, including patients with more advanced tumor stages (T3–4), is warranted in the future.
Conclusions
For small, well-lateralized oral-cavity cancers, low contralateral neck nodal recurrence rates were observed. The benefit of contralateral neck irradiation cannot be well established according to our study. Once local failure occurs, cautionary follow-up is necessary because it may indicate a higher likelihood of contralateral neck failure. Further prospective, multi-center randomized controlled trials are warranted.
Acknowledgments
We kindly acknowledge the kind assistance of Candace W. for wording advice of the manuscript.
Funding: This research was supported by a grant NCKUH-11103011 from the National Cheng Kung University Hospital.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tro.amegroups.com/article/view/10.21037/tro-22-27/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of National Cheng Kung University Hospital (IRB number: A-ER-111-181). The requirement for informed consent from the study subjects was waived due to the retrospective study design.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lin CY, Fan KH, Lee LY, et al. Precision Adjuvant Therapy Based on Detailed Pathologic Risk Factors for Resected Oral Cavity Squamous Cell Carcinoma: Long-Term Outcome Comparison of CGMH and NCCN Guidelines. Int J Radiat Oncol Biol Phys 2020;106:916-25. [Crossref] [PubMed]
- Liu HY, Tam L, Woody NM, et al. Failure rate in the untreated contralateral node negative neck of small lateralized oral cavity cancers: A multi-institutional collaborative study. Oral Oncol 2021;115:105190. [Crossref] [PubMed]
- Wirtz MM, Temming S, Kocher M, et al. Low risk of contralateral lymph node recurrence in lateralized head and neck carcinoma after postoperative ipsilateral radiotherapy. Strahlenther Onkol 2020;196:474-84. [Crossref] [PubMed]
- Lin CY, Lee LY, Huang SF, et al. Treatment outcome of combined modalities for buccal cancers: unilateral or bilateral neck radiation? Int J Radiat Oncol Biol Phys 2008;70:1373-81. [Crossref] [PubMed]
- Al-Mamgani A, van Werkhoven E, Navran A, et al. Contralateral regional recurrence after elective unilateral neck irradiation in oropharyngeal carcinoma: A literature-based critical review. Cancer Treat Rev 2017;59:102-8. [Crossref] [PubMed]
- Capote-Moreno A, Naval L, Muñoz-Guerra MF, et al. Prognostic factors influencing contralateral neck lymph node metastases in oral and oropharyngeal carcinoma. J Oral Maxillofac Surg 2010;68:268-75. [Crossref] [PubMed]
- Greene FL, Page DL, Fleming ID, et al. editors. AJCC cancer staging manual, 6th edh New York, NY: Springer; 2002.
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Contreras JA, Spencer C, DeWees T, et al. Eliminating Postoperative Radiation to the Pathologically Node-Negative Neck: Long-Term Results of a Prospective Phase II Study. J Clin Oncol 2019;37:2548-55. [Crossref] [PubMed]
Cite this article as: Lee CH, Wu YH, Hsueh WT, Pao TH, Cheng YJ. Contralateral neck nodal outcomes and recurrence pattern of small well-lateralized oral cavity carcinoma—a single institution experience. Ther Radiol Oncol 2023;7:1.


