
Patients with cervical cancer without visceral obesity had better treatment outcomes
Introduction
According to data provided by the World Health Organization in 2018, cervical cancer is the fourth most common cancer in women worldwide (1). Data from the Health Promotion Administration, Ministry of Health and Welfare, indicate that cervical cancer is the eighth most common cause of cancer-related death in women in Taiwan in 2013 (2). Based on current knowledge, risk factors for developing cervical cancer include human papillomavirus infection, a history of multiple sexual partners, and cigarette smoking. However, obesity is a health problem associated with the development of malignant tumors. Studies have examined the relationship between obesity [mostly measured by body mass index (BMI)] and cancer risk and treatment outcomes (3-5). A meta-analysis revealed that obesity is mildly associated with an increased risk of cervical cancer (4). In Taiwan, nearly 50.6% of people are overweight (defined by the government as a BMI of ≥24), and 21.1% of people are obese (BMI ≥27). Moreover, approximately 81.5% of women are obese (as defined by a female body fat percentage of ≥30%) (6).
Visceral adipose mass may be a more accurate measure of the dysfunctional adipose tissue that affects cancer development and progression than BMI (3). Britton et al. assessed the fat distribution of over 3,000 patients through multidetector-computed tomography (CT) and revealed that visceral adiposity relates to increased incidence of cardiovascular disease and cancer (7). Therefore, increases in visceral adipose tissue are more likely than subcutaneous adipose tissue to cause obesity-related diseases or cancer. Biological mechanisms may contribute to insulin resistance and chronic inflammation (8-10).
CT is the gold-standard method for obtaining quantitative radiologic measures of adipose tissue (11,12), particularly for analyzing visceral fat. Studies have analyzed the relationship between visceral fat and cancer outcomes. Clark et al. identified 99 rectal adenocarcinoma patients and measured their visceral and subcutaneous fat areas with CT scans, which indicated that the visceral fat-subcutaneous fat (V/S) ratio predicted rectal cancer prognosis (3). Nevertheless, studies on obesity and cervical cancer outcomes have been rare. Therefore, we studied the relationship between body fat composition and treatment outcomes in patients with cervical cancer. We hypothesized that the V/S ratio identified by a CT scan prior to definitive concurrent chemoradiation therapy is associated with survival outcomes in patients with cervical cancer. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tro-20-22).
Methods
Study design and participants
This retrospective cohort study collected data of cervical cancer (stage I–III) patients treated with definitive concurrent chemoradiotherapy (CCRT) from 2010 to 2013. The last follow up time was on May, 2019. The patients were treated with chemotherapy involving weekly administration of cisplatinum (50 mg/m2) or oral tegafur/uracil (UFUR; 200 mg) twice per day. All patients received external beam radiation with intensity-modulated radiation therapy, along with the simultaneous integrated boost technique. The gross tumor volume (GTV) involved the gross cervical tumor, uterus, cervix, and gross regional lymph nodes. The clinical target volume (CTV) included the pelvic and regional lymph nodes. The planning target volume (PTV) was the GTV or CTV plus a 5-mm margin, named PTV-GTV or PTV-CTV, respectively. All patients had received brachytherapy at doses of 1,350 to 2,750 cGy prescribed to point-A for 3–5 times. The total dose to PTV-GTV was 5,700 to 6,120 cGy (180–200 cGy per fraction). The dose to PTV-CTV was 4,500 to 4,680 cGy (180 cGy per fraction). Other parameters were retrieved from medical records, including patients’ age; performance status with Eastern Cooperative Oncology Group (ECOG) criteria; whether patients had more than two comorbidities, type 2 diabetes mellitus (DM), or hypertension; clinical T and N stages; and histologic type and grade. In our medical records, comorbidities included hypertension, type 2 DM, asthma, cerebrovascular disease, cardiovascular disease, and hepatitis. We focused on hypertension and type 2 DM because hypertension was the most common comorbidity in our patient data, and type 2 DM may be caused by obesity and affect treatment outcomes. The last follow up date was May, 2019. All the patients were followed up for at least 5 years. This study was approved by the Institutional Review Board (IRB) of Chung Shan Medical University Hospital (IRB number: CS19076). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The patients were not required to give informed consent before participating in the study because the research involves no more than minimal risk to subjects.
Adiposity measures
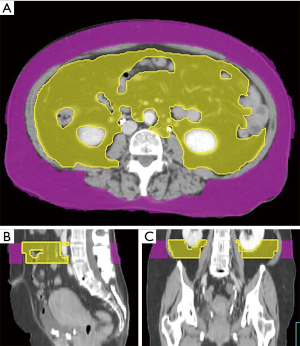
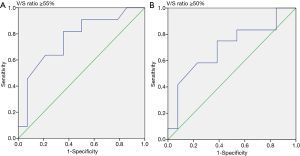
We collected the patients’ CT images during the CT simulation process, by using a Philips Brilliance CT Big Bore (Philips, Amsterdam, Netherlands). The scanning process was performed at a standard tube voltage of 120 kV, with the corresponding tube current–time product settings being 300 mAs. The slice thickness was 5 mm. Radiological measurements of adipose tissue were obtained from the CT images. Visceral and subcutaneous fat areas were defined as the volume of adipose tissue measured from axial slices at the L4 level. The fat areas were contoured from the top to the bottom of L4 (Figure 1). The adipose tissue regions were delineated, and the total volume of visceral fat area, subcutaneous fat area, V/S ratio over L4 were calculated to measure abdominal fat by using Pinnacle 8.0, Philips. The adipose tissue was contoured by the senior resident doctors in our department, and two radiation oncologists with over 5 and 15 years of experience checked the contour volume. We calculated the ROC (receiver operating characteristic) curve to set the V/S ratio threshold of 0.55 for defining visceral obesity (Figure 2). A V/S ratio of ≥0.55 was considered to indicate visceral obesity.
Statistical analysis
The patients were divided into 2 groups: groups 1, comprising patients with a V/S ratio of ≥55%; and group 2, comprising those with a V/S ratio of <55%. Fisher’s exact test was applied to compare the categorical variables. Overall survival (OS) was defined as the time from diagnosis until death from any cause. OS rates were estimated using the Kaplan-Meier method. The statistical significance of the effect of RT on all end points was tested using the log-rank test. A P value of <0.05 was considered to indicate statistical significance. All analyses were conducted using SAS (Statistical Analysis System) version 9.4 (SAS Institute, Cary, NC, USA).
Results
Clinical characteristics of patients
Our study included 25 women, all of whom were diagnosed as having stage I–III cervical cancer and received CCRT from 2010 to 2013 in Chung Shan Medical University Hospital. All of the patients were followed up for at least 5 years. Table 1 presents the baseline and clinical characteristics of patients in group 1 and group 2. Their ages at diagnosis ranged from 31 to 88 years, with the median age being 62 years. Of the 25 patients, 21 (84%) fulfilled ECOG performance status criteria of 0 or 1, and 4 (16%) fulfilled ECOG performance status criteria 2 or 3. For comorbidity, most patients (76%) had fewer than two comorbidities before starting CCRT. Only 2 (8%) patients had type 2 DM, and 8 (32%) had hypertension. In total, 4 (16%) were diagnosed as having T stage Ib1/Ib2 cancer, 19 (76%) as having T stage 2a/2b cancer, and 2 (8%) as having T stage 3a cancer. A total of 20 patients (80%) with squamous cell carcinoma and 2 patients (8%) with adenocarcinoma were included. Lymph node metastasis was observed in 7 patients (28%). Through histologic grading, we assessed 23 (92%) and 2 (8%) patients as having G1/G2 and G3 tumors, respectively. Most of the patients (96%) received chemotherapy with weekly cisplatin and radiotherapy (RT) at a dose of more than 6,000 cGy. All patients received intracavitary brachytherapy. Moreover, 11 (44%) patients had a V/S ratio of ≥55%, and 14 (56%) patients had a V/S ratio of <55%. Patients’ age in group 1 was significantly higher than group 2.
Table 1
| Characteristic | Patient numbers | Group 1 (V/S ratio ≥55%) | Group 2 (V/S ratio <55%) | P value |
|---|---|---|---|---|
| Age | 0.005 | |||
| ≥65 | 12 | 9 | 3 | |
| <65 | 13 | 2 | 11 | |
| Performance status | 0.288 | |||
| ECOG 0 and 1 | 21 | 8 | 13 | |
| ECOG 2 and 3 | 4 | 3 | 1 | |
| Comorbidities | 1.000 | |||
| ≥2 | 6 | 3 | 3 | |
| <2 | 19 | 8 | 11 | |
| Type 2 DM | 0.288 | |||
| Yes | 2 | 1 | 1 | |
| No | 23 | 10 | 13 | |
| Hypertension | 0.241 | |||
| Yes | 8 | 4 | 4 | |
| No | 17 | 7 | 10 | |
| T stage | 0.223 | |||
| Ib1/Ib2 | 4 | 2 | 2 | |
| 2a/2b | 19 | 7 | 12 | |
| 3a | 2 | 2 | 0 | |
| Lymph node metastasis | 0.407 | |||
| Yes | 7 | 2 | 5 | |
| No | 18 | 9 | 9 | |
| Histology type | 0.262 | |||
| Squamous cell carcinoma | 20 | 10 | 10 | |
| Adenocarcinoma | 2 | 0 | 2 | |
| Others | 3 | 1 | 2 | |
| Grade | 0.250 | |||
| G1/G2 | 18 | 7 | 11 | |
| G3 | 2 | 2 | 0 | |
| Other | 5 | 2 | 3 | |
| Chemotherapy | 1.000 | |||
| Weekly cisplatin | 24 | 11 | 13 | |
| Oral UFUR | 1 | 0 | 1 | |
| Radiotherapy dose | 1.000 | |||
| ≥6,000 cGy | 24 | 11 | 13 | |
| <6,000 cGy | 1 | 0 | 1 | |
| Brachytherapy dose | 1.000 | |||
| ≥2,500 cGy | 19 | 8 | 11 | |
| <2,500 cGy | 6 | 3 | 3 | |
| Total patients | 25 | 11 | 14 |
G1, well-differentiated tumor; G2, moderately differentiated tumor; G3, poorly differentiated tumor.
Correlation between clinical characteristics and OS
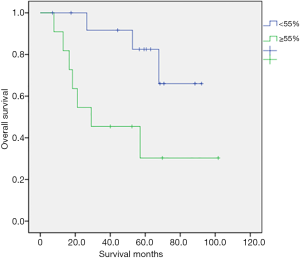
Table 2 shows the univariate analysis results for OS in 25 patients with various clinical characteristics. We observed a statistically significant association between OS and V/S ratio (P=0.021). By contrast, no significant relationships were noted between OS and age, performance status with ECOG criteria; whether patients had more than two comorbidities, type 2 DM, hypertension, lymph node metastasis, histologic type, histologic grade, chemotherapy regimen, RT dose, or brachytherapy dose. Although Group 1 was significantly older, age did not significantly influence the OS. Figure 3 illustrates the OS curves. The 5-year survival rate was 60% in all patients. The 5-year survival rate was 82.5% in group 2 and 30.3% in group 1 (P=0.021). Group 2 had a longer OS. The median survival time was 30 months in all patients. There were 10 patients died at the last follow up period, with seven patients in group 1 and three patients in group 2. In group 1, four patients died because of the disease and three were not. Every patient who died in group 2 were due to the disease.
Table 2
| Variables | Patient numbers | Overall survival (months) | P value |
|---|---|---|---|
| Age | |||
| ≥65 | 12 | 60.37 | 0.326 |
| <65 | 13 | 71.11 | |
| Performance status | |||
| ECOG 0 and 1 | 21 | 51.44 | 0.263 |
| ECOG 2 and 3 | 4 | 30.58 | |
| Comorbidities | |||
| ≥2 | 6 | 46.42 | 0.916 |
| <2 | 19 | 48.62 | |
| Type 2 DM | |||
| Yes | 2 | 39.94 | 0.344 |
| No | 23 | 49.65 | |
| Hypertension | |||
| Yes | 8 | 44.42 | 0.566 |
| No | 17 | 50.55 | |
| T stage | |||
| Ib1/Ib2 | 4 | 74.87 | 0.758 |
| 2a/2b | 19 | 64.76 | |
| 3a | 2 | 29.23 | |
| Lymph node metastasis | |||
| Yes | 7 | 50.2 | 0.628 |
| No | 18 | 71.37 | |
| Histology type | |||
| Squamous cell carcinoma | 20 | 70.84 | 0.344 |
| Adenocarcinoma | 2 | 52.7 | |
| Others | 3 | – | |
| Grade | |||
| G1/G2 | 18 | 73.59 | 0.427 |
| G3 | 2 | 26.57 | |
| Other | 5 | – | |
| Chemotherapy | |||
| Cisplatin | 24 | – | 0.712 |
| UFUR | 1 | – | |
| Radiotherapy dose | |||
| ≥6,000 cGy | 24 | – | 0.712 |
| <6,000 cGy | 1 | – | |
| Brachytherapy | |||
| ≥2,500 cGy | 19 | 71.81 | 0.693 |
| <2,500 cGy | 6 | 53.25 | |
| V/S ratio | |||
| ≥55% | 11 | 49.12 | 0.021 |
| <55% | 14 | 79.06 | |
G1, well-differentiated tumor; G2, moderately differentiated tumor; G3, poorly differentiated tumor.
Visceral-to-subcutaneous adipose volume
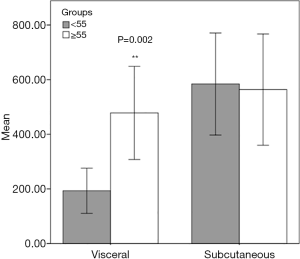
We analyzed the V/S ratio (Figure 4). We determined that subcutaneous fat volumes in groups 1 and 2 were similar. However, the visceral fat levels in both groups were significantly different (P=0.002). The visceral fat volume was significantly larger in group 1. Therefore, visceral fat exerted greater effects on the V/S ratio than did subcutaneous fat.
Discussion
To the best of our knowledge, this is the first study to assess the V/S ratio as prognostic factor of cervical cancer. A high V/S ratio was associated with an increased risk of death. This association was not observed with other clinical features such as age, histologic grade, histologic type, or lymph node metastasis. Our study determined the relationship between visceral obesity and treatment outcomes among patients with cervical cancer.
Previous studies have examined the association of obesity, measured by BMI, and cancer risk (3-5). Specifically, a previous study conducted a meta-analysis to explore the association between BMI and cervical cancer risk (4); the study concluded that overweight is not associated with an increased risk of cervical cancer, but obesity is mildly associated with an increased risk of cervical cancer. However, whether obesity may affect cancer incidence is relatively inconclusive. In addition, Choi et al. assessed the effect of BMI on the outcomes of cervical cancer patients (5). They concluded that overweight or obese (BMI ≥23 kg/m2) patients with cervical cancer had poorer 5-year cancer-specific survival than did other patients. Clark et al. studied the relationship between BMI and rectal cancer outcomes (3). They set a BMI cutoff point of >30 kg/m2 to define obesity and revealed that disease-free survival and OS were not affected by obesity when defined by BMI. BMI represents only the height-to-weight ratio; it cannot represent body fat. Furthermore, BMI does not distinguish between visceral and subcutaneous fat and is not an accurate measurement of abdominal obesity. Therefore, we chose to measure visceral adipose mass rather than BMI for OS analysis.
Apart from BMI, several studies have revealed that body fat distribution, abdominal obesity in particular, is a risk factor for cancer. Studies have presented abdominal obesity in various ways, which can be measured by outside body surface such as waist circumference or inside body fat such as visceral fat. Ogundiran et al. reported findings from the Nigerian Breast Cancer Study, which involved 1,233 individuals with invasive breast cancer and 1,101 community controls and measured abdominal obesity by waist circumference. This study found that the waist-hip ratio was associated with increased breast cancer risk (13). Song et al. conducted a prospective study to measure abdominal adiposity by using waist circumference, hip circumference, and waist–hip ratio. They noted that abdominal adiposity, independent of overall obesity, was associated with an increased risk of colorectal cancer in men (14). Britton et al. assessed the fat distribution of visceral, pericardial, and periaortic adipose tissue through multidetector CT (7). They analyzed 3,086 patients from the Framingham Heart Study Offspring cohort. They discovered that visceral adiposity was associated with increasing risk of cardiovascular disease and cancer.
Studies have analyzed abdominal obesity measured by visceral fat or visceral fat ratio, which is related to cancer outcomes. Mauland et al. reported that a higher visceral fat volume percentage (VAV%) was associated with older age (P<0.001) and reduced disease-specific survival (P=0.041) in endometrial cancer (15). They concluded that a high VAV% can independently predict reduced endometrial cancer survival. Moreover, Nattenmüller et al. conducted CT to measure visceral abdominal fat as a prognostic factor for gynecologic cancer (16). They reported increased incidence of gynecologic cancer in obese people. Nevertheless, they determined no significant effect of body composition, including visceral adipose tissue, on patient survival. In these studies, there were a large variation of the absolute volume of the adipose tissue. We calculated the ratio between the visceral and the subcutaneous fat to decrease the variation. The present study revealed that among patients with cervical cancer who were treated with definitive CCRT, those with a V/S ratio of <55% had significantly longer OS than did those with a V/S ratio of ≥55%.
Clark et al. (3) indicated that the V/S ratio predicts rectal cancer prognosis. They examined patients with rectal cancer who received neoadjuvant chemoradiation. The results indicated that elevated the V/S ratio was associated with shorter disease-free survival (P=0.02) and OS times (P=0.047). The visceral and subcutaneous fat areas were measured from a single axial slice at the L4–L5 intervertebral space. By contrast, we derived the cumulative visceral fat volume from contours of visceral and subcutaneous fat at the L4 level.
During the past decades, evidence has linked obesity with increased cancer incidence and prognosis (17,18). Obesity at diagnosis may also be associated with cancer mortality (19,20). The relationship between cancer and obesity is complex. Obesity and adipose tissue may lead to metabolic changes and affect cancer cells or tumor microenvironments (21,22). Biological mechanisms linking adiposity to poor prognosis of tumors include insulin, insulin-like growth factors, glucose, cytokines, adipokines, local and systemic inflammation, and steroid hormones. Systemic obesity and local adipose tissue can affect cancer directly by activating key signal pathways and changing cell metabolisms, which may involve glucose, free fatty acids, and lipids. Adipose tissue may also affect tumor microenvironments by promoting tumor proliferation, angiogenesis, and invasion (23-25). In our study, patients with a higher V/S ratio (Group 1) had poor OS, which may be related to metabolic changes in tumor microenvironments, such as from chronic inflammation.
This study has several limitations. First, the sample size was relatively small. Thus, we calculated post hoc power to be 0.88. The high power of this result indicates the viability of further research. Moreover, we continuously followed up the patients to ensure the accuracy of limited information for over 5 years. Second, the data were collected from 2010 to 2013, which may have presented a time period bias. However, we noted no change in the cervical cancer treatment protocol from 2010 to 2013; hence, the bias was reduced. Third, this was a retrospective study. Patients’ stages and received RT doses were slightly different, but all of them had met the treatment guidelines. This study involved preliminary data and is a starting point for future studies. We are initiating another cohort study containing more patients with cervical and other cancers.
In conclusion, OS was longer in group 2. This may be a prognostic factor for patients with stage I–III cervical cancers who receive definitive CCRT. Additional studies with larger cohorts are necessary.
Acknowledgments
We thank the patients that participated in this study. We are grateful to the Cancer Registration Center of Chung Shan Medical University Hospital for collecting data. This manuscript was edited by Wallace Academic Editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tro-20-22
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro-20-22). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board (IRB) of Chung Shan Medical University Hospital (IRB number: CS19076). The patients were not required to give informed consent before participating in the study because the research involves no more than minimal risk to subjects.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Organization WH. Cervical Cancer. 2019. Available online: https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/
-
Taiwan Health Promotion Administration MoHaW 2015 . Available online: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=269&pid=10227 - Clark W, Siegel EM, Chen YA, et al. Quantitative measures of visceral adiposity and body mass index in predicting rectal cancer outcomes after neoadjuvant chemoradiation. J Am Coll Surg 2013;216:1070-81. [Crossref] [PubMed]
- Poorolajal J, Jenabi E. The association between BMI and cervical cancer risk: a meta-analysis. Eur J Cancer Prev 2016;25:232-8. [Crossref] [PubMed]
- Choi Y, Ahn KJ, Park SK, et al. Adverse effect of excess body weight on survival in cervical cancer patients after surgery and radiotherapy. Radiat Oncol J 2017;35:48. [Crossref] [PubMed]
-
Taiwan Health Promotion Administration MoHaW 2013 . Available online: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=364&pid=6543 - Britton KA, Massaro JM, Murabito JM, et al. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol 2013;62:921-5. [Crossref] [PubMed]
- Goossens GH. The metabolic phenotype in obesity: fat mass, body fat distribution, and adipose tissue function. Obes Facts 2017;10:207-15. [Crossref] [PubMed]
- Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol 2019;15:139-54. [Crossref] [PubMed]
- Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med 2016;375:794-8. [Crossref] [PubMed]
- Gradmark AM, Rydh A, Renström F, et al. Computed tomography-based validation of abdominal adiposity measurements from ultrasonography, dual-energy X-ray absorptiometry and anthropometry. Br J Nutr 2010;104:582-8. [Crossref] [PubMed]
- Pou KM, Massaro JM, Hoffmann U, et al. Patterns of abdominal fat distribution: the Framingham Heart Study. Diabetes Care 2009;32:481-5. [Crossref] [PubMed]
- Ogundiran TO, Huo D, Adenipekun A, et al. Body fat distribution and breast cancer risk: findings from the Nigerian breast cancer study. Cancer Causes Control 2012;23:565-74. [Crossref] [PubMed]
- Song M, Hu FB, Spiegelman D, et al. Long-term status and change of body fat distribution, and risk of colorectal cancer: a prospective cohort study. Int J Epidemiol 2015;45:871-83. [Crossref] [PubMed]
- Mauland KK, Eng Ø, Ytre-Hauge S, et al. High visceral fat percentage is associated with poor outcome in endometrial cancer. Oncotarget 2017;8:105184 [Crossref] [PubMed]
- Nattenmüller J, Rom J, Buckner T, et al. Visceral abdominal fat measured by computer tomography as a prognostic factor for gynecological malignancies? Oncotarget 2018;9:16330. [Crossref] [PubMed]
- De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes 2013;2013:291546 [Crossref] [PubMed]
- Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N Y Acad Sci 2012;1271:37. [Crossref] [PubMed]
- Parekh N, Chandran U, Bandera EV. Obesity in cancer survival. Annu Rev Nutr 2012;32:311-42. [Crossref] [PubMed]
- Demark-Wahnefried W, Platz EA, Ligibel JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomarkers Prev 2012;21:1244-59. [Crossref] [PubMed]
- Iyengar NM, Gucalp A, Dannenberg AJ, et al. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol 2016;34:4270. [Crossref] [PubMed]
- Hopkins BD, Goncalves MD, Cantley LC. Obesity and cancer mechanisms: cancer metabolism. J Clin Oncol 2016;34:4277. [Crossref] [PubMed]
- Ellulu MS, Patimah I, Khaza’ai H, et al. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci 2017;13:851. [Crossref] [PubMed]
- Divella R, De Luca R, Abbate I, et al. Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. J Cancer 2016;7:2346. [Crossref] [PubMed]
- Feola A, Ricci S, Kouidhi S, et al. Multifaceted breast cancer: the molecular connection with obesity. J Cell Physiol 2017;232:69-77. [Crossref] [PubMed]
Cite this article as: Chen HL, Shih CT, Lee YC, Tseng HC, Chou YH. Patients with cervical cancer without visceral obesity had better treatment outcomes. Ther Radiol Oncol 2020;4:27.


