
Prognostic classification for patients with nasopharyngeal carcinoma based on American Joint Committee on cancer staging system T and N categories
Introduction
Nasopharyngeal carcinoma (NPC) is an uncommon disease worldwide, with high prevalence in endemic regions such as South China and Southeast Asia. Nearly all cases in endemic areas are non-keratinizing subtypes and are related to the Epstein-Barr virus (EBV). Radiotherapy is the preferred treatment modality for NPC because of its high radiosensitivity; moreover, its anatomical location is unsuitable for surgery, except in cases requiring salvage therapy. The Intergroup 0099 study first showed that concurrent, adjuvant chemotherapy improved the outcomes of patients with NPC (1). Further studies from endemic areas have also demonstrated the efficacy of concurrent chemoradiotherapy (CRT) (2-5). Several other factors, including advanced conformal radiotherapy techniques, progress in diagnostic imaging, novel systemic agents, and improved medical care, have also improved the prognosis of NPC. A novel staging system is warranted to reflect these improved outcomes.
The American Joint Committee on Cancer (AJCC) TNM staging system is one of the most commonly used staging systems that provides a strategy for grouping patients with respect to their prognosis. Compared to the previous edition, the current 8th edition staging system has been validated to provide more accurate prognostic information (6). However, our previous studies have demonstrated that selected patients with stage III NPC treated with CRT without adjuvant chemotherapy had a 10-year overall survival (OS) rate of 90% (7,8). Given the good prognosis in selected patients with stage III disease, this study examined the current staging system and attempted to allocate patients into more homogeneous groups that reflect their favorable prognosis.
Methods
Study population and data collection
With institutional review board (IRB) approval, we retrospectively analyzed newly diagnosed patients with histological confirmation of NPC treated at our institution from 1995 to 2010. The Consolidated Standards of Reporting Trials (CONSORT) flow diagram is presented in Figure 1. One thousand five hundred twenty five newly diagnosed patients with NPC were identified in our NPC database. Patients with prior cancer history or metastatic disease were excluded. Finally, 1,418 patients remained for the training and validation analyses. Patients who received three-dimensional conformal radiotherapy (3D-CRT) were used as the training cohort. Patients who received intensity-modulated radiation therapy (IMRT) were used as the validation cohort. IMRT was first implemented in our department in the third quarter of 2003.

According to IRB guidelines, we collected the clinical information of all patients prospectively. The comprehensive data of all patients are organized and stored in the NPC database as reported previously (9). SAS programs were used to manage quality control and data input (10). Pretreatment evaluation included detailed medical history, physical and neurological examination, nasopharyngoscopy, complete blood counts, and serum chemistry panels. Magnetic resonance imaging (MRI) and/or computerized tomography (CT) of the head and neck region was used to evaluate the extent of the disease. Chest X-ray, bone scintigraphy or positron emission tomography CT (PET-CT), and liver sonography were used to assess whether there was any distant metastases.
Treatment and surveillance
Diagnostic images of individual patients were reviewed by a multidisciplinary team. The anatomical structures invaded by the primary tumor and the regions of metastatic lymph nodes were recorded. The criteria for diagnosing lymph node metastases included >5 mm for retropharyngeal lymph nodes, >10 mm in the shortest axis for cervical lymph nodes, lymph nodes with contrast-enhanced rims or central necrosis, suspicious contiguous and confluent lymph nodes near significant lymph nodes, and lymph nodes with fluorodeoxyglucose (FDG) uptake if 18F-FDG PET-CT was performed.
The treatment strategy for each patient involved a multidisciplinary approach and followed institutional guidelines. Generally, stage I (T1N0M0, AJCC 7th edition) patients were treated solely with radiation therapy. Patients with low-risk stage II–III NPC (T1–T2a disease) were treated with CRT without adjuvant chemotherapy. Patients with intermediate-risk stage II–III NPC (T2b–T3 disease) underwent CRT and adjuvant chemotherapy. Stage IVA–B patients were treated with CRT followed by adjuvant chemotherapy and weekly maintenance fluorouracil (5-FU) and leucovorin for 6 months (11).
All patients were followed-up at intervals of 3 months for the first 2 years, intervals of 6 months for the third through fifth years, and 1-year intervals thereafter. Nasopharyngoscopy was performed at each clinic visit. MRI of the head and neck region was performed 3 months after the completion of radiotherapy to evaluate the treatment response.
Statistical analysis
We compared the differences between the training and validation cohorts using Fisher’s exact test for categorical variables. The Kruskal-Wallis test was used to compare the differences between continuous variables. The primary outcome was OS, which was defined as the time from treatment to death from any cause and was calculated using the Kaplan-Meier method (12). A log-rank test was used to evaluate statistical significance in specific patient subsets (13). Hazard ratios (HRs) for risk of death were calculated using multivariable Cox regression (14). We treated the T (T1–T4) and N (N0–N3) categories of the AJCC 7th edition staging system for NPC as ordinal variables. Factors included in the multivariate analysis were age, sex, and the use of concurrent chemotherapy. We calculated the Harrell concordance index (C-index) by using the Survival package in R (15). The C-index assesses the discrimination power of a model (16). The range of the C-index was between 0.5 and 1, and better predictive performance is indicated by a higher C-index. To compare the goodness of the fit of the model, we also calculated the Akaike information criterion (AIC) (17). AIC can estimate the relative amount of information lost by a model. A lower AIC indicates better performance of a model.
We assumed that our new staging system could be universally applied regardless of radiation technique (e.g., 3D-CRT or IMRT). Patients treated with 3D-CRT were used as the training cohort, and those treated with IMRT were used as the validation cohort. Recursive partitioning analysis (RPA) was used to reclassify the combinations of the AJCC 7th edition T and N categories according to the similarity of their outcomes in the training cohort. The HRs for the risks of death for the 7th edition T category, N category, and each T and N combination were calculated and adjusted by age, sex, and the use of concurrent chemotherapy. After RPA classification, we compared the performance of the RPA-derived model with that of the AJCC 8th edition staging system in the validation cohort. All analyses were performed using R version 3.5.2 (R Foundation for Statistical Computing).
Results
The median follow-up period was 84.6 months (range, 2–175 months). Clinical and demographic characteristics are provided in Table 1. Among all patients, 71.4% were men. The median age was 45 years (range, 10–86 years). Stage distribution according to the 8th edition staging system was as follows: 95 patients in stage I (6.7%); 226 patients in stage II (15.9%); 609 patients in stage III (42.9%) and 488 patients in stage IVA (34.4%)
Table 1
| Characters | Total (n=1,418) | 3D-CRT (n=643) | IMRT (n=775) | P value |
|---|---|---|---|---|
| Sex, n (%) | 0.516 | |||
| Male | 1,012 (71.4) | 453 (70.5) | 559 (72.1) | |
| Female | 406 (28.6) | 190 (29.5) | 216 (27.9) | |
| Age, median [range], years | 45 [10–86] | 44 [10–86] | 45 [14–78] | 0.62 |
| The 7th edition T category, n (%) | <0.001 | |||
| T1 | 491 (34.6) | 212 (33.0) | 279 (36.0) | |
| T2 | 214 (15.1) | 133 (20.7) | 81 (10.5) | |
| T3 | 375 (26.4) | 136 (21.2) | 239 (30.8) | |
| T4 | 338 (23.8) | 162 (25.2) | 176 (22.7) | |
| The 7th edition N category, n (%) | 0.0851 | |||
| N0 | 171 (12.1) | 76 (11.8) | 95 (12.3) | |
| N1 | 361 (25.5) | 152 (23.6) | 209 (27.0) | |
| N2 | 731 (51.6) | 354 (55.1) | 377 (48.6) | |
| N3 | 155 (10.9) | 61 (9.5) | 94 (12.1) | |
| Treatment, n (%) | 0.00817 | |||
| CRT | 1,357 (95.7) | 605 (94.1) | 752 (97.0) | |
| RT | 61 (4.3) | 38 (5.9) | 23 (3.0) | |
| The 7th edition stage, n (%) | 0.41 | |||
| I | 95 (6.7) | 45 (7.0) | 50 (6.5) | |
| II | 232 (16.4) | 108 (16.8) | 124 (16.0) | |
| III | 644 (45.4) | 287 (44.6) | 357 (46.1) | |
| IVA | 292 (20.6) | 142 (22.1) | 150 (19.4) | |
| IVB | 155 (10.9) | 61 (9.5) | 94 (12.1) | |
| The 8th edition stage, n (%) | 0.95 | |||
| I | 95 (6.7) | 45 (7.0) | 50 (6.5) | |
| II | 226 (15.9) | 105 (16.3) | 121 (15.6) | |
| III | 609 (42.9) | 273 (42.5) | 336 (43.4) | |
| IV | 488 (34.4) | 220 (34.2) | 268 (34.6) |
Fisher’s exact test for categorical variables was used to compare the differences between the training and validation cohorts. The Kruskal-Wallis test was used to compare continuous variables. T and N categories were analyzed based on the AJCC 7th edition staging system. AJCC, American Joint Committee on Cancer; 3D-CRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiation therapy; CRT, concurrent chemoradiotherapy; RT, radiotherapy.
For the entire cohort, the 5-year OS rate was 92.6% [95% confidence interval (CI), 87.4–98.0] for the AJCC 8th edition stage I patients, 94.1% (95% CI, 91.0–97.3%) for stage II patients, 86.9% (95% CI, 84.3–89.7%) for stage III patients, and 72.0% (95% CI, 68.0–76.1%) for stage IVA patients (Figure 2A). The 5-year OS for patients with AJCC 8th edition T1, T2, T3, and T4 NPC were 90.2% (95% CI, 87.6–92.9%), 85.8% (95% CI, 81.2–90.6%), 82.6% (95% CI, 79.1–86.4%), and 70.5% (95% CI, 65.4–76.1%). The 5-year OS rate for patients with 8th edition N0, N1, N2, or N3 NPC were 88.8% (95% CI, 84.1–93.7%), 90.2% (95% CI, 87.1–93.5%), 82.8% (95% CI, 79.9–85.8%), and 71.8% (95% CI, 66.5–77.5%) (Figure 2B,C). In the multivariate Cox regression analyses adjusted by age, sex, and the use of concurrent chemotherapy, the 8th edition stage II disease did not differ significantly from stage I disease (HR, 1.5; 95% CI, 0.8–2.9; P=0.198). Age and sex (males had lower survival rate) affected the OS in patients with NPC (Table 2).
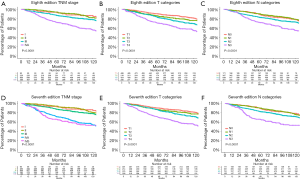
Table 2
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| AJCC stage I | 1 (reference) | – |
| AJCC stage II | 1.53 (0.8–2.9) | 0.195 |
| AJCC stage III | 2.38 (1.29–4.4) | 0.005 |
| AJCC stage IVA | 4.87 (2.69–8.8) | <0.001 |
| Age | 1.046 (1.04–1.1) | <0.001 |
| Sex (male |
1.28 (1.02–1.6) | 0.03 |
| RT |
1.73 (0.99–3.0) | 0.053 |
AJCC, American Joint Committee on Cancer; CI, confidence interval; CRT, concurrent chemoradiotherapy; RT, radiotherapy.
The 5-year OS rate was 92.6% (95% CI, 87.4–98.0%) for the AJCC 7th edition stage I patients, 94.2% (95% CI, 91.2–97.3%) for stage II patients, 87.6% (95% CI, 85.1–90.2%) for stage III patients, 71.4% (95% CI, 66.4–76.9%) for stage IVA patients, and 65.5% (95% CI, 58.3–73.6%) for stage IVB patients (Figure 2D). The 5-year OS for patients with AJCC 7th edition T1, T2, T3, and T4 NPC were 90.1% (95% CI, 87.4–92.8%), 86.4% (95% CI, 81.9–91.2%), 85.0% (95% CI, 81.4–88.7%), and 69.7% (95% CI, 64.9–74.8%). The 5-year OS rate for patients with 7th edition N0, N1, N2, or N3 NPC were 88.8% (95% CI, 84.1–93.7%), 90.0% (95% CI, 87.0–93.2%), 82.5% (95% CI, 79.7–85.3%), and 65.5% (95% CI, 58.3–73.6%) (Figure 2E,F). Multivariate analyses using Cox regression models adjusted by age, sex, and the use of concurrent chemotherapy revealed no significant differences between the 7th edition T1, T2, and T3 categories and no significant differences between the N0 and N1 categories (Table 3).
Table 3
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| T category (the 7th edition) | ||
| T1 | 1 (reference) | – |
| T2 | 1.00 (0.72–1.4) | 0.977 |
| T3 | 1.25 (0.94–1.6) | 0.12 |
| T4 | 2.35(1.83–3.0) | <0.001 |
| N category (the 7th edition) | ||
| N0 | 1 (reference) | – |
| N1 | 1.10 (0.74–1.6) | 0.637 |
| N2 | 1.58 (1.10–2.3) | 0.014 |
| N3 | 2.90 (1.91–4.4) | <0.001 |
| Age | 1.048 (1.04–1.1) | <0.001 |
| Sex (male |
1.29 (1.04–1.6) | 0.023 |
| RT |
1.33 (0.8–2.2) | 0.267 |
AJCC, American Joint Committee on Cancer; CI, confidence interval; CRT, concurrent chemoradiotherapy; RT, radiotherapy.
Table 4 shows the HRs for the risks of mortality according to combinations of the 7th edition T and N categories adjusted by age, sex, and the use of concurrent chemotherapy. In the validation dataset, patients with T2N2 and T3N2 disease had significantly higher HRs [4.37 (95% CI, 1.07–17.8) and 4.03 (95% CI, 1.19–13.6), respectively] than did patients with T1N0 disease. The HRs for the risks of mortality are higher in patients with T4 or N3 disease, which are 6.62 (95% CI, 1.41–31.0), 5.69 (95% CI, 1.56–20.7), 8.02 (95% CI, 2.33–27.6), 7.72 (95% CI, 1.98–30.1), 13.22 (95% CI, 3.52–49.6), 7.33 (95% CI, 1.56–34.4), 7.91 (95% CI, 2.09–29.9) for patients with T4N0, T4N1, T4N2, T4N3, T1N3, T2N3 and T3N3 NPC, respectively.
Table 4
| AJCC 7th edition T-N categories | T1 | T2 | T3 | T4 |
|---|---|---|---|---|
| Training cohort | ||||
| N0 | 1, n=45 | 1.03 (0.29–3.7), n=11 | 0.89 (0.11–7.1), n=6 | 3.71 (1.52–9.1), n=14 |
| N1 | 1.58 (0.71–3.5), n=54 | 1.09 (0.44–2.7), n=43 | 1.27 (0.47–3.5), n=26 | 2.97 (1.31–6.7), n=29 |
| N2 | 1.39 (0.64–3.0), n=100 | 1.65 (0.74–3.6), n=66 | 2.73 (1.29–5.8), n=89 | 3.60 (1.78–7.3), n=99 |
| N3 | 4.29 (1.57–11.7), n=13 | 2.94 (1.08–8.0), n=13 | 2.35 (0.86–6.4), n=15 | 8.49 (3.66–19.7), n=20 |
| Validation cohort | ||||
| N0 | 1, n=50 | 2.06 (0.33–12.9), n=10 | 2.94 (0.67–12.9), n=26 | 6.62 (1.41–31.0), n=9 |
| N1 | 1.84 (0.49–7.0), n=93 | 0.82 (0.08–8.1), n=21 | 2.02 (0.48–8.5), n=48 | 5.69 (1.56–20.7), n=47 |
| N2 | 2.81 (0.78–10.1), n=109 | 4.37 (1.07–17.8), n=36 | 4.03 (1.19–13.6), n=138 | 8.02 (2.33–27.6), n=94 |
| N3 | 13.22 (3.52–49.6), n=27 | 7.33 (1.56–34.4), n=14 | 7.91 (2.09–29.9), n=27 | 7.72 (1.98–30.1), n=26 |
AJCC, American Joint Committee on Cancer.
Among the 643 patients with NPC treated with 3D-CRT, the RPA algorithm divided the patients into 3 groups. Group I included patients with T1–3N0–1 and T1N2 disease, group II included those with T2–3N2 disease, and group III included those with T4 or N3 disease. The 5-year OS rate were 92.3%, 82.5%, and 65.4% for each group, respectively (Figure 3, Figure 4A,B).
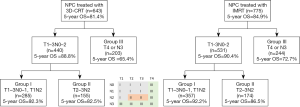
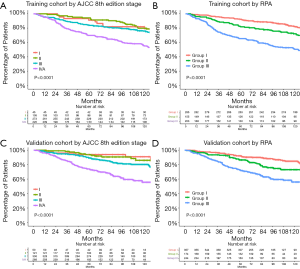
For the validation cohort (IMRT group, n=775), the 5-year OS rate according to the AJCC 8th edition staging system were 94.0% for stage I (n=50), 93.9% for stage II (n=121), 89.2% for stage III (n=336), and 73.7% for stage IVA (n=268). After RPA classification, the 5-year OS rate in the validation cohort were 92.2% for group I (n=357), 86.5% for group II (n=174), and 72.7% for group III (n=244) (Figure 3, Figure 4C,D). Table 5 shows the HRs for the risks of death using the RPA-derived grouping system, adjusted for age, sex, and the use of concurrent chemotherapy for the training, validation, and entire cohorts.
Table 5
| RPA group | Hazard ratio (95% CI) | P value |
|---|---|---|
| Training cohort | ||
| Group I | 1 (reference) | – |
| Group II | 1.67 (1.18–2.4) | 0.004 |
| Group III | 2.84 (2.13–3.8) | <0.001 |
| Validation cohort | ||
| Group I | 1 (reference) | – |
| Group II | 1.95 (1.27–3.0) | 0.002 |
| Group III | 3.66 (2.55–5.3) | <0.001 |
| Total | ||
| Group I | 1 (reference) | – |
| Group II | 1.80 (1.37–2.4) | <0.001 |
| Group III | 3.22 (2.57–4.0) | <0.001 |
RPA, recursive partitioning analysis; CI, confidence interval.
The C-index for the validation cohort was 0.6934 for the RPA-derived group and 0.6930 for the 8th edition staging system after adjusting for age, sex, and the use of concurrent chemotherapy. The AIC was 2,142.96 for the RPA-derived group and 2,141.33 for the 8th edition staging system. Therefore, the performances were similar between the two models.
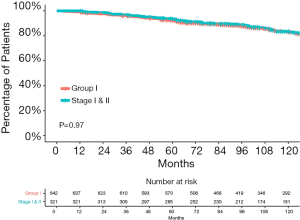
Compared with the current staging system, more patients were considered to have early-stage disease in the entire cohort according to the RPA-derived grouping system (group I vs. the 8th edition stage I and II: 45.3% vs. 22.6%, P<0.001). Figure 5 shows that the survival curves of the RPA-group I patients from the entire cohort and the 8th edition stage I and II patients were not significantly different (P=0.79). The 5-year OS rate were 92.3% for group I patients and 93.6% for the 8th edition stage I and II patients.
Discussion
Using our institutional prospective NPC database, we were able to demonstrate that patients with NPC treated using modern techniques have excellent outcomes. Patients with the 8th edition stage I and II disease achieved equally good outcomes. The 5-year OS rate of patients with stage II disease was higher than that of patients with stage I disease in the entire cohort (94.1% vs. 92.6%). In addition, our data showed that selected patients with stage III disease (T3N0–1 and T1N2) had good prognosis with 5-year survival rate of more than 90%. Therefore, it is not necessary to divide these patients into different prognostic groups; the current staging system still lacks the power of prognostic discrimination (Table 2).
Our proposed RPA grouping system better reflects the favorable prognosis of NPC in the modern era, and more patients are considered to have early disease. Classified as group I by RPA, T1N2 and T3N0 disease would be classified as stage III disease according to the current AJCC 8th edition staging system. The diagnosis of “stage III” disease may cause unnecessary panic in patients, even if the treatment outcomes are excellent. Our findings are valuable as they have the potential to reduce emotional distress in these patients.
We also found that N0 and N1 categories had similar risks of death in our cohort. Limited lymph node metastasis does not seem to affect the survival outcomes of patients with NPC in the modern era. Moreover, the risk of death in the AJCC 7th edition T1–3 categories did not differ significantly (Table 3). The 5-year OS rate were similar for patients with T2 and T3 disease; however, the survival curves diverged after 5 years which may be attributed to mortality related to late toxicities from treatment rather than a difference in the prognosis of T2 and T3 disease (Figure 2B,C,D,E). Tang et al. also demonstrated that there was no significant difference in locoregional failure-free survival, disease-free survival, and OS between T2 and T3 disease in the era of IMRT (6). Another study showed that T3 patients with pterygoid process or sphenoid bone base invasion had better prognosis than those with invasion in other sites in the base of the skull (18).
The T category of the current staging system is primarily based on the treatment outcomes of 2-dimensional radiotherapy (2D-RT) using opposed bilateral portals without CT simulation. It is challenging to achieve sufficient target coverage without harming normal tissues in advanced disease with this outdated technique. With advancements in radiotherapy techniques, the survival outcomes for NPC have improved substantially due to improvements in target volume coverage and normal tissue sparing. The use of systemic agents and progress in diagnostic imaging have also contributed to improved prognosis. Lee et al. demonstrated improved survival in patients with NPC treated with 3D-CRT and IMRT compared to those treated with 2D-RT (19). The main differences occurred in patients with stage III–IV disease; the 5-year survival rate of these patients was 60% in the 2D-RT era and 79% in the IMRT era. The late toxicity rate has also improved in the IMRT era. However, more advanced T and N categories are associated with increased volumes in high-dose areas which could lead to more treatment-related toxicities.
One meta-analysis demonstrated an increased risk of carotid artery stenosis in patients with NPC treated with radiotherapy (20), which can lead to stroke or even death. Radiation-associated dysphagia is caused by damage to the anatomic structures associated with swallowing, such as the pharyngeal constrictor muscles, and may lead to aspiration pneumonia. Although damage to normal tissues caused by radiation decreased with the advent of IMRT, late treatment-related toxicities may become more severe over time and become a competing risk of death. Efforts should be made to reduce the incidence of radiation-associated late toxicities by reducing treatment intensity, especially for patients with a life expectancy of more than 5 years.
Several ongoing trials are designed to test the feasibility of treatment de-escalation for patients with human papillomavirus-related oropharyngeal cancer. Given good outcomes in the modern era, treatment de-escalation may also be considered for patients with early-stage NPC to reduce the incidence of late treatment-related toxicities. A meta-analysis demonstrated that, for patients with stage II NPC, concurrent chemotherapy did not improve OS, but increased acute grade 3 or 4 toxicities compared to radiation therapy alone in the IMRT era (21). The lack of a survival benefit may be due to improved locoregional control with IMRT. A combined analysis of the NPC-9901 and NPC-9902 trials demonstrated that, for patients with stage III–IV NPC, there was no significant difference in disease control between patients receiving either two or three concurrent cycles of cisplatin (100 mg/m2) (22). Decreasing the intensity of chemotherapy may be considered as a strategy for treatment de-escalation in patients with good prognosis. Another prospective randomized study revealed that reducing the target volume after induction chemotherapy subsequently reduced the radiation dose received by healthy tissues and did not compromise the 3-year outcome for patients with stage III–IV NPC (23). The quality of life was also improved. More phase III studies are necessary to demonstrate the feasibility of treatment de-escalation for patients with NPC.
Even with modern treatment, the prognosis of patients with T4 or N3 NPC remains suboptimal. The 5-year OS rate of the patients in group III in our study was only 69.4% (65.4% in the 3D-CRT group and 72.7% in the IMRT group). Treatment intensification may be considered for these patients, and strategies to intensify treatments in the current practice include induction and/or adjuvant systemic therapy. Recently, several studies have shown the benefits of induction chemotherapy prior to concurrent CRT for patients with locally-advanced NPC. A randomized controlled phase 3 study from China has demonstrated that, for patients with locally-advanced NPC, induction gemcitabine and cisplatin (GC) followed by cisplatin-based CRT improved OS and recurrence-free survival compared to those of patients treated with CRT alone (24). The main benefit of induction GC was the improvement in 3-year distant-metastasis recurrence-free survival (HR, 0.43; 91.1% in the induction GC followed by CRT group vs. 84.4% in the CRT alone group). There was no significant difference in 3-year locoregional recurrence-free survival. The benefits were more pronounced for patients with N2–3 diseases compared to patients with N1 disease. According to the results of this study, for patients with advanced N-category NPC, induction GC should be considered as a treatment strategy.
Another randomized control study from Taiwan revealed that, for patients with stage IVA–IVB NPC (AJCC 7th edition), induction chemotherapy with three cycles of cisplatin, epirubicin, mitomycin C, and 5-FU/leucovorin (MEPFL) prior to CRT improved disease-free survival compared to that of patients treated with CRT alone (25). The 5-year disease-free survival rate were 61% in the induction arm and 50% in the CRT alone arm. In contrast to induction GC, the main benefit of induction MEPFL was the improvement in locoregional failure-free survival (HR, 0.664; 5-year rate 80% in the induction MEPFL followed by CRT group vs. 70% in the CRT alone group). There was no difference in OS or distant failure-free survival between the two arms. Further research is necessary to determine the optimal treatment for these patients. Novel agents including immune checkpoint inhibitors may have the potential to improve prognosis for patients with locally advanced NPC.
One strength of our study is that it was based on a large cohort of patients in a prospective database. We also demonstrated that our RPA-derived model was applicable to patients treated with 3D-CRT and IMRT. However, there are several limitations to our study. The first is the retrospective design of the analysis, which may have led to several forms of bias. The second is that the data were from a single institution with its own protocol; further external validation is needed to test its generalizability. Moreover, some known prognostic factors for NPC, including World Health Organization classification, lactate dehydrogenase level, performance status, and plasma EBV DNA level, were not considered in the survival analysis. Furthermore, we analyzed survival outcomes but did not analyze other important endpoints, including locoregional-free survival, distant metastasis-free survival, and non-cancer-related death. An analysis based on these endpoints may help optimize treatment strategies for NPC. However, we demonstrated more favorable outcomes for patients with NPC. Therefore, a new staging system for NPC based on modern treatment methodologies is urgently needed.
In this single-center retrospective cohort study, we demonstrated that the current staging system does not reflect the favorable survival outcomes of patients with NPC in the modern era. By using RPA, we propose a novel grouping system for NPC as follows: group I (AJCC 7th edition T1–3N0–1 and T1N2), group II (T2–3N2), and group III (T4 or N3). Patients with metastatic disease can be classified into group IV and have the worst prognosis. Based on this grouping system, more patients with NPC are considered to have early-stage disease with favorable outcomes. Further external validation is warranted to confirm the performance of this grouping system.
Acknowledgments
We are thankful for the efforts of every member in the head and neck multidisciplinary team.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2020.02.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All study participants provided informed consent, and the study design was approved by the appropriate ethics review board.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 1998;16:1310-7. [Crossref] [PubMed]
- Chen Y, Liu MZ, Liang SB, et al. Preliminary results of a prospective randomized trial comparing concurrent chemoradiotherapy plus adjuvant chemotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma in endemic regions of china. Int J Radiat Oncol Biol Phys 2008;71:1356-64. [Crossref] [PubMed]
- Lee AW, Lau WH, Tung SY, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol 2005;23:6966-75. [Crossref] [PubMed]
- Lee AW, Tung SY, Chan AT, et al. Preliminary results of a randomized study (NPC-9902 Trial) on therapeutic gain by concurrent chemotherapy and/or accelerated fractionation for locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2006;66:142-51. [Crossref] [PubMed]
- Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol 2005;23:6730-8. [Crossref] [PubMed]
- Tang LL, Chen YP, Mao YP, et al. Validation of the 8th Edition of the UICC/AJCC Staging System for Nasopharyngeal Carcinoma From Endemic Areas in the Intensity-Modulated Radiotherapy Era. J Natl Compr Canc Netw 2017;15:913-9.
- Wu JS, Tsai YC, Jian JJ, et al. Survival without adjuvant chemotherapy for selected patients with stage II and III nasopharyngeal carcinoma after concurrent chemoradiotherapy alone. Head Neck 2018;40:2070-7. [Crossref] [PubMed]
- Cheng SH, Yen KL, Jian JJ, et al. Examining prognostic factors and patterns of failure in nasopharyngeal carcinoma following concomitant radiotherapy and chemotherapy: impact on future clinical trials. Int J Radiat Oncol Biol Phys 2001;50:717-26. [Crossref] [PubMed]
- Cheng SH, Tsai SY, Yen KL, et al. Concomitant radiotherapy and chemotherapy for early-stage nasopharyngeal carcinoma. J Clin Oncol 2000;18:2040-5. [Crossref] [PubMed]
- Cheng SH, Prosnitz LR. Utilizing Clinical and Genomic Information to Select Patients for Postmastectomy Radiotherapy. Am J Hematol Oncol 2007;6:213-6.
- Cheng SH, Tsai SY, Yen KL, et al. Prognostic significance of parapharyngeal space venous plexus and marrow involvement: potential landmarks of dissemination for stage I-III nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2005;61:456-65. [Crossref] [PubMed]
- Kaplan EL MP. Nonparametric estimation from incomplete observation. J Am Stat Assoc 1958;53:457-81. [Crossref]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966;50:163-70. [PubMed]
- Cox DR. Regression models and life-tables. J R Stat Soc 1972;34:187-220.
- Therneau TM (2015). A Package for Survival Analysis in S.
- Harrell FE Jr, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA 1982;247:2543-6. [Crossref] [PubMed]
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control 1974;19:716-23. [Crossref]
- Li HJ, Hu YY, Huang L, et al. Subclassification of skull-base invasion for nasopharyngeal carcinoma using cluster, network and survival analyses: A double-center retrospective investigation. Radiother Oncol 2019;134:37-43. [Crossref] [PubMed]
- Lee AW, Ng WT, Chan LL, et al. Evolution of treatment for nasopharyngeal cancer--success and setback in the intensity-modulated radiotherapy era. Radiother Oncol 2014;110:377-84. [Crossref] [PubMed]
- Liao W, Zheng Y, Bi S, et al. Carotid stenosis prevalence after radiotherapy in nasopharyngeal carcinoma: A meta-analysis. Radiother Oncol 2019;133:167-75. [Crossref] [PubMed]
- Liu F, Jin T, Liu L, et al. The role of concurrent chemotherapy for stage II nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: A systematic review and meta-analysis. PLoS One 2018;13:e0194733 [Crossref] [PubMed]
- Ng WT, Tung SY, Lee V, et al. Concurrent-Adjuvant Chemoradiation Therapy for Stage III-IVB Nasopharyngeal Carcinoma-Exploration for Achieving Optimal 10-Year Therapeutic Ratio. Int J Radiat Oncol Biol Phys 2018;101:1078-86. [Crossref] [PubMed]
- Yang H, Chen X, Lin S, et al. Treatment outcomes after reduction of the target volume of intensity-modulated radiotherapy following induction chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: A prospective, multi-center, randomized clinical trial. Radiother Oncol 2018;126:37-42. [Crossref] [PubMed]
- Zhang Y, Chen L, Hu GQ, et al. Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. N Engl J Med 2019;381:1124-35. [Crossref] [PubMed]
- Hong RL, Hsiao CF, Ting LL, et al. Final results of a randomized phase III trial of induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with stage IVA and IVB nasopharyngeal carcinoma-Taiwan Cooperative Oncology Group (TCOG) 1303 Study. Ann Oncol 2018;29:1972-9. [Crossref] [PubMed]
Cite this article as: Jen CW, Tsai YC, Wu JS, Chen PL, Yen JH, Chuang WK, Cheng SHC. Prognostic classification for patients with nasopharyngeal carcinoma based on American Joint Committee on cancer staging system T and N categories. Ther Radiol Oncol 2020;4:2.


