
Sorafenib-triggered radiation recall dermatitis: case report and literature review
IntroductionOther Section
Radiation recall dermatitis (RRD) is characterized by an acute inflammatory dermatological reaction confined to previously irradiated skin after the precipitating agent is administered. RRD is a poorly understood and unpredictable phenomenon with several possible mechanisms proposed (1). RRD could be triggered by a variety of both antineoplastic and non-antineoplastic agents and could occur weeks to years after radiotherapy (2-5).
Sorafenib is a multi-kinase inhibitor targeting several serine/threonine and receptor tyrosine kinases. The United States Food and Drug Administration has approved sorafenib for the treatment of patients with advanced renal cell carcinoma (RCC), unresectable hepatocellular carcinoma (HCC), and locally recurrent or metastatic, progressive differentiated thyroid carcinoma (DTC) refractory to radioactive iodine treatment.
As the technique of radiotherapy advances, we can expect the combination of Sorafenib and radiotherapy more commonly seen in clinical practice. Recognizing RRD is of clinical importance to provide adequate management and improve patients’ quality of life.
Sorafenib-induced radiation recall dermatitis is rare. The incidence and epidemiology is unknown. We largely rely on published case reports to further explore its pathogenesis and prevalence. There were only 8 reported cases of sorafenib-induced radiation recall reactions (RRR) with systemic search in PubMed (“sorafenib”, “radiation recall”) when we completed the current case report (1,6-12). Seven of the reported cases are confined to dermatologic reaction while one describes radiation recall reaction causing cardiotoxicity. All of the eight reported cases are male patients. Here we would like to present the first female patient of sorafenib-induced radiation recall dermatitis previously irradiated for bone metastasis from HCC.
Case presentationOther Section
The patient was an 86-year-old Taiwanese woman. She had medical history as the following description: (I) chronic hepatitis C and liver cirrhosis, (II) moderately differentiated adenocarcinoma of transverse colon, cT3N1M0, clinical stage group IVA (according to AJCC 7th), post right hemicolectomy on 2009-01-07, pT3N0, pathologic stage group IIA (according to AJCC 7th); (III) type 2 diabetes mellitus; (IV) Hypertension.
She received regular abdominal sonographic examination in our hospital every 6 months after colon cancer surgery. Eight months later, a hyperechoic nodule in liver segment 8, with estimated size of 1.6 cm, was detected. Her serum alpha-fetoprotein level was elevated (6.2 ng/mL). The patient decided to receive observation first instead of further investigation or intervention. A month later, new occurrence of liver segment 5 tumor with 2.3 cm in size, and increasing size of prior S8 tumor were found by triphasic computed tomography scan. Secondary HCC, clinical T2N0M0, stage II, was diagnosed clinically combining classic image features, elevated serum alpha-fetoprotein level (29.48 ng/mL) and further staging workup. She was treated with transarterial chemoembolization (TACE) once followed by 6 times of radiofrequency ablation (RFA) in the next four months.
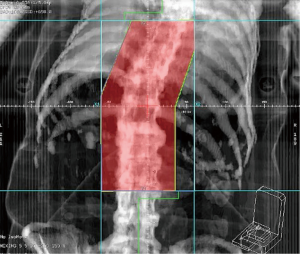
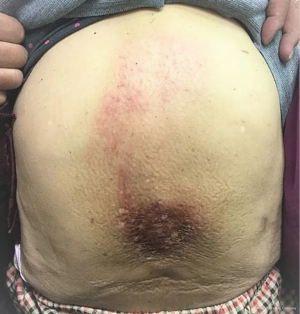
With clinical complete response of liver tumors, she kept regular follow-up per 3–4 months in our institution afterwards without evidence of recurrent disease for 2 years. Then she presented severe lower back pain for 2 weeks, caused by bone metastases confirmed by Tc-99m bone scan. A recurrent HCC over segment 5 of liver was found by abdominal Magnetic Resonance Imaging. No obvious recurrent tumor was noted before in the follow-up sonography. Her serum alpha-fetoprotein level remained within normal range (≤9 ng/mL) and elevated to 12.27 ng/mL at the diagnosis of recurrence. Radiotherapy to symptomatic bone metastases including T8-9 spine with radiation dose of 3,000 cGy in 10 fractions was delivered by 6 MV photon beam using 2D single posterior-anterior (PA) field technique, prescribed to the depth of 5.5 cm. The radiation treatment field was shown in Figure 1. There was no adverse event after the initiation and during the radiotherapy until a month after completion of radiotherapy, when sorafenib 400 mg was administered orally twice daily for her metastatic disease. Progressive pruritus occurred and restricted to the previous irradiated skin of lower back. One week after starting sorafenib, the patient presented with grade 1 pruritus, erythema, desquamation and hyperpigmentation without pain (CTCAE v4.03), all confined to previous irradiated skin area (Figure 2). There was no skin reaction prior to starting sorafenib.
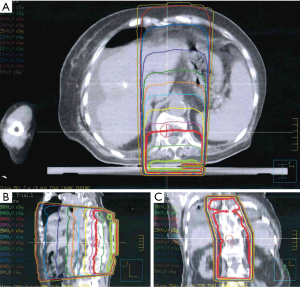
The intensity of skin reaction was not homogenous with a more severe reaction confined to skin folds, compared to the upper and middle part of the irradiated field where the surface was flattened with relatively mild skin reaction within the higher dose curve of 3,800 cGy (Figures 3,4). The dose to the front of the chest was 500–1,000 cGy without any skin reaction after starting sorafenib.
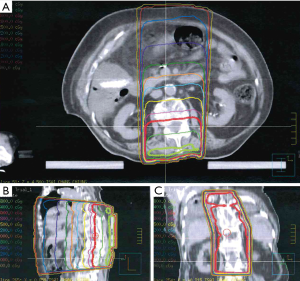
The clinical presentation was consistent with recall radiation dermatitis. Topical steroid cream was prescribed for the dermatologic symptoms without adjusting the dose of sorafenib. Her skin reaction resolved in several days later with no other adverse reactions.
DiscussionOther Section
Radiation recall phenomenon was first described by D’Angio et al. triggered by actinomycin D in 1959 (2). Radiation recall dermatitis is drug-specific and unpredictable. Common RRD-eliciting agents include anthracyclines, taxanes, etoposide, gemcitabine, vinorelbine, ertolinib, cetuximab, trastuzumab, and other non-anticancer drugs such as antibiotics and anti-tuberculosis drugs.
The clinical presentation of radiation recall dermatitis has been described as: hyperpigmentation, pruritus, erythema, desquamation, erythematous rash, and eczematous dissemination, which differs between each individual (1,6,7,9,12). The severity of skin reaction varies and there might be a time- and radiation dose-dependency. A minimum threshold of radiation dose to skin with 7 Gy is proposed to cause recall dermatological reaction (9). The associated radiation doses ranges from 20 to 70 Gy in reported sorafenib-triggered RRD. The dose of Sorafenib varies from 200 mg twice daily to 400 mg twice daily.
Although not fully understood, several possible mechanisms of radiation recall dermatitis has been proposed: (I) It is possible that stem cells in the irradiated area have increased sensitivity to subsequent chemotherapy. (II) Another hypothesis of cutaneous radiation recall reaction involves activation of nonimmune inflammatory pathways. Irradiation lowers the threshold of inflammatory response and induces continued low-level secretion of the inflammation-mediating cytokines. The administration of a promoting agents may upregulate these cytokines and then causes a radiation recall reaction. (III) The histologic features suggest that keratinocyte necrosis plays a major role in radiation recall. The mechanism is related to increased oxidative stress and direct DNA damage caused by specific chemotherapeutic agents, which leads to accelerating cellular necrosis (3,4,13).
The pathological finding of sorafenib-induced skin reaction can show both keratinocyte damage of varying degrees as in sorafenib-induced skin reaction and inflammatory reaction with wound healing process as in acute radiation dermatitis (14,15). Stieb et al. reported a skin biopsy of RRD caused by sorafenib and the histological finding mimicked graft-versus-host reactions of the skin or cutaneous drug allergies, suggesting that the mechanism might be immune-mediated (12). In our case, the clinical condition did not justify a skin biopsy and therefore it was not performed.
Sorafenib is an oral multi-kinase inhibitor targeting several serine/threonine and receptor tyrosine kinases. Sorafenib was shown to interact with multiple intracellular (CRAF, BRAF and mutant BRAF) and cell surface kinases (KIT, FLT-3, VEGFR-2, VEGFR-3, and PDGFR-β). Several of these kinases are thought to be involved in angiogenesis. VEGF would be increased in a time- and dose-dependent manner after sublethal irradiation damage to HCC cells in vitro, leading to enhanced intratumor angiogenesis in vivo and correlating well with serum VEGF (16). Sorafenib might enhance the efficacy of radiation, when administered following radiation. Current standard treatment indicates sorafenib can be considered in selected patients based on two randomized phase 3 clinical trials that have demonstrated survival benefits (17,18). The Taiwan Food and Drug Administration has approved sorafenib in current clinical practice for the treatment of patients with advanced RCC, advanced HCC, and locally advanced or metastatic, progressive differentiated thyroid carcinoma refractory to radioactive iodine treatment (RAI-R DTC).
Skin reactions are among the most common adverse reactions of sorafenib. When combined with radiation, sorafenib could present the radiosensitizing effect (19). Potential photosensitivity of sorafenib has been reported by Hsieh et al. describing a case of RCC with photo-induced recall dermatitis secondary to sorafenib (20).
Chung and colleagues reported in 2010 the first case of sorafenib-triggered radiation recall dermatitis after completing radiotherapy to the liver metastases (1). We summarize the characteristics of previously reported sorafenib-triggered RRD in Table 1. The average age is 58.7 years, including the current case. The total dose of radiotherapy ranges from 20 to 70 Gy. Most of the sorafenib-induced RRD happens within 1 to 2 weeks after the beginning of sorafenib therapy.
Table 1
| Author, year | Age | Gender | Dx | RT | Dosage | Onset after RT | Onset after sorafenib | Symptoms | Treatment | Adjustment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Current study | 86 | Female | HCC | Bone metastases 30 Gy in 10 fx | 400 mg BID | 33 days | 1 day | Hyperpigmentation, pruritus, erythema, desquamation | Topical steroids | continued | Dermatologic reaction resolved in several days |
| Mehta, 2018 | 59 | Male | HCC | Right forearm metastatic lesion 30 Gy in 10 fx | 400 mg BID | 21 days | 7 days | Marked erythema and dry desquamation in the previously irradiated area with sharp demarcation of the adjacent skin | Topical antibiotics and topical steroids | discontinued | Dermatitis improved shortly afterwards |
| Kim, 2017 | 44 | Male | HCC | Right 7th rib mass 30 Gy in 10 fx | 400 mg BID | 8 days | 11 days | Erythematous patch with pruritus (dermatitis CTCAE grade 1) in the right 7th anterior rib area | No medical therapy | continued | RRD subsided over 42 days after radiotherapy |
| Stieb, 2015 | 77 | Male | HCC | Bone metastases 20–36 Gy | 400 mg BID | 2 - 59 days | 10 -17 days | Erythematous papules and plaques, eczematous reaction with severe pruritus (dermatitis CTCAE grade 1–3) | Topical steroids and oral antihistamines | discontinued | Skin reaction decreased within 2 weeks |
| Masri, 2014 | 56 | Male | Lung cancer | Chest and cervical lymph nodes 70 Gy | N/A | N/A | 5 months | Chest pain, decreased left ventricular systolic function, small pericardial effusion, edema of basal and mid anterior wall of heart | No medical therapy | discontinued | No recurrent chest pain after sorafenib was discontinued |
| Hsieh, 2014 | 63 | Male | HCC | Liver SBRT 8 Gy × 6 fx | 300 mg BID | 17 days | 1 week | RRD grade 2 | Topical steroids | discontinued | Symptoms resolved after |
| Oh, 2013 | 50 | Male | HCC | Chest wall mass 3 Gy × 13 fx | 400 mg BID | 2 weeks | 2 weeks | Erythematous patch, dry desquamation, dissemination, pruritus | Oral antihistamines | discontinued | Sorafenib was restarted and no recurrent RRD |
| Robbins, 2011 | 55 | Male | HCC | L2 spine metastatic mass 20 Gy × 1 fx | 400 mg BID | 8 weeks | 2 weeks | Dry, itchy, flaky skin and redness, several patchy erythematous band-like lesions with dry desquamation | No medical therapy | N/A | N/A |
| Chung, 2010 | 38 | Male | HCC | Liver SBRT 5 Gy × 6 fx | 200 mg BID | Sorafenib administered 3 weeks after completing RT. | Several days | Progressive pruritus, fatigue, patchy hyperpigmentation, dry desquamation | Topical steroids | continued | Pruritus and skin changes resolved after 2–3 weeks, Sorafenib was escalated without exacerbation of RRD |
RT, radiotherapy; Dx, Diagnosis; CTCAE, Common Terminology Criteria for Adverse Events; Gy, gray; HCC, hepatocellular carcinoma; N/A, not available; Dosage, daily sorafenib dosage; Onset after RT, Onset of RRD after completing RT; Onset after sorafenib, Onset of RRD after starting sorafenib; Adjustment, Adjustment of sorafenib; BID, twice daily; fx, fraction.
The presentation of our case also supports the proposed threshold dose for radiation recall dermatitis being isodose 20 Gy in literature review. The skin with RRD generally received more than 20 Gy while no skin reaction was observed in lower dose areas (less than 10 Gy in anterior chest wall). The severity of RRD could be affected by radiation dose. Our patient had more severe reaction at the bottom part. Combing the fact that the bottom of irradiated filed was not perpendicular to the beam and the patient had more loose skin in her lower back, we believe the phenomenon could be explained by hotspots caused by the propensity for higher doses of radiation to reach the skin folds.
The time interval between completing radiotherapy and the administration of triggering agents might aid in differentiating radiation recall dermatitis from the radiosensitization effect as later occurrence (e.g., >7 days) is observed in RRD (14). The likelihood of developing RRD seems to be decreased as the time interval between radiotherapy and exposure to RRD-eliciting agents.
The management of RRD includes either decreasing the dose or discontinuing of eliciting agents with systemic steroid therapy, topical treatment, or antihistamines. However, in our case, sorafenib was continued at the same dose after evaluation. After topical steroid cream was used, the dermatologic reaction resolved within days. In cases that sorafenib was discontinued, the severity of skin reaction and the clinical condition should be evaluated before re-administration. The re-administration of sorafenib has not been reported to trigger the recurrence of RRD.
Here we present a case of recall radiation dermatitis one week after starting sorafenib, with RRD of skin matched previously irradiated area of T8-9 spine. Given the fact that the skin reaction occurred 33 days after completing radiotherapy and confined to irradiated skin area, the diagnosis of RRD is more likely than either acute radiation dermatitis or adverse skin reaction of sorafenib.
In the GLOBOCAN 2018 estimated new cases of cancer incidence, 29% of liver cancer, 36.9% of kidney cancer, and 76.9% of thyroid cancer are females. Yet the current literature of sorafenib-triggered RRD does not reflect the gender distribution. RRD in women caused by other agents has been reported. To the best of our knowledge, this is so far the first case report of a female patient presenting sorafenib-triggered RRD. The mechanism of RRD has yet to be fully disclosed. Our case report highlights the possibility of sorafenib-triggered RRD in both genders and could provide more information. Although it is premature to make assumption about the reason behind gender distribution in reported literature, clinicians should be alert to the possibility of RRD in patients with dermatologic reaction in previously irradiated skin after receiving sorafenib.
In conclusion, radiation recall dermatitis induced by sorafenib is an unpredictable and not thoroughly understood phenomenon. The recognition of this phenomenon may aid in providing adequate and timely management.
AcknowledgmentsOther Section
Funding: None.
FootnoteOther Section
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The patient has died and thus is unable to provide consent. Consent would be unusually burdensome to obtain from her family. We have removed all information which could possibly be used to identify the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Chung C, Dawson LA, Joshua AM, et al. Radiation recall dermatitis triggered by multi-targeted tyrosine kinase inhibitors: sunitinib and sorafenib. Anticancer Drugs 2010;21:206-9. [Crossref] [PubMed]
- D'Angio GJ, Farber S, Maddock CL. Potentiation of x-ray effects by actinomycin D. Radiology 1959;73:175-7. [Crossref] [PubMed]
- Azria D, Magne N, Zouhair A, et al. Radiation recall: a well recognized but neglected phenomenon. Cancer Treat Rev 2005;31:555-70. [Crossref] [PubMed]
- Burris HA 3rd, Hurtig J. Radiation recall with anticancer agents. Oncologist 2010;15:1227-37. [Crossref] [PubMed]
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol 2006;54:28-46. [Crossref] [PubMed]
- Hsieh CH, Lin SC, Shueng PW, et al. Recall radiation dermatitis by sorafenib following stereotactic body radiation therapy. Onco Targets Ther 2014;7:1111-4. [Crossref] [PubMed]
- Oh D, Park HC, Lim HY, et al. Sorafenib-triggered radiation recall dermatitis with a disseminated exanthematous reaction. Radiat Oncol J 2013;31:171-4. [Crossref] [PubMed]
- Kim GE, Song HS, Ahn KJ, et al. Radiation recall dermatitis triggered by sorafenib after radiation therapy for hepatocellular carcinoma. Radiat Oncol J 2017;35:289-94. [Crossref] [PubMed]
- Robbins J, Wollner I, Ryu S. Sorafenib induced radiation recall dermatitis after spine radiosurgery. J Radiosurg SBRT 2011;1:71-4. [PubMed]
- Mehta K, Kaubisch A, Tang J, et al. Radiation Recall Dermatitis in Patients Treated with Sorafenib. Case Rep Oncol Med 2018;2018:2171062 [Crossref] [PubMed]
- Masri SC, Misselt AJ, Dudek A, et al. Radiation recall reaction causing cardiotoxicity. J Cardiovasc Magn Reson 2014;16:25. [Crossref] [PubMed]
- Stieb S, Riesterer O, Brussow C, et al. Radiation recall dermatitis induced by sorafenib: A case study and review of the literature. Strahlenther Onkol 2016;192:342-8. [Crossref] [PubMed]
- Smith KJ, Germain M, Skelton H. Histopathologic features seen with radiation recall or enhancement eruptions. J Cutan Med Surg 2002;6:535-40. [Crossref] [PubMed]
- Camidge R, Price A. Characterizing the phenomenon of radiation recall dermatitis. Radiother Oncol 2001;59:237-45 [Crossref] [PubMed]
- Yang CH, Lin WC, Chuang CK, et al. Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol 2008;158:592-6. [Crossref] [PubMed]
- Chung YL, Jian JJ, Cheng SH, et al. Sublethal irradiation induces vascular endothelial growth factor and promotes growth of hepatoma cells: implications for radiotherapy of hepatocellular carcinoma. Clin Cancer Res 2006;12:2706-15. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Huang CY, Lin CS, Tai WT, et al. Sorafenib enhances radiation-induced apoptosis in hepatocellular carcinoma by inhibiting STAT3. Int J Radiat Oncol Biol Phys 2013;86:456-62. [Crossref] [PubMed]
- Hsieh CH, Jeng KS, Lin CC, et al. Combination of sorafenib and intensity modulated radiotherapy for unresectable hepatocellular carcinoma. Clin Drug Investig 2009;29:65-71. [Crossref] [PubMed]
Cite this article as: Lin FY, Chang TH, Huang CC, Hung LC, Chou TW, Lin JB. Sorafenib-triggered radiation recall dermatitis: case report and literature review. Ther Radiol Oncol 2019;3:39.


