Radiotherapy of malignant pheochromocytoma—a case report
Introduction
Pheochromocytomas (PCC) and paragangliomas (PGL) are rare catecholamine-secreting tumors that arise from chromaffin cells of the adrenal medulla and the sympathetic ganglia respectively. This tumor is usually located in the abdomen (98%), and more specifically in the adrenal medulla (90%) (1). This same tumor when found in extrarenal sites such as the neck, mediastinum, pelvis, and organ of Zuckerkandl is termed as PGL (2). The prevalence of PCC is increasing due to advancement in imaging technology, resulting in a high rate of incidental adrenal mass (3).
Catecholamine-secreting tumors are rare, with an estimated incidence of 0.4 to 9.5 cases per 1 million per year (4). There are approximately 500 to 1,600 new cases in the United States per year (5).
In 1886, Felix Fränkel (6,7) was the first to describe a PCC patient. He described an 18-year-old female patient who presented with fast, irregular and very shallow pulse, intermittent attacks of palpitation, anxiety, vertigo, headache, chest pain, cold sweats, and vomiting. The patient died 10 days after hospital admission. At autopsy, bilateral adrenal tumors were noted and were initially diagnosed as angiosarcomas, but later a positive chromaffin reaction confirmed PCC.
The term PCC was derived from the Greek words phaios (“dusky”), chroma (“color”), and cytoma (“tumor”). The dark staining reaction of PCC tumor was caused by the oxidation of intracellular catecholamines when stained with dichromate salts. Epinephrine and norepinephrine were later isolated from PCC in 1936 and in 1949 respectively (7).
Multiple endocrine neoplasia syndromes 2A or 2B, von Hippel-Lindau syndrome, and neurofibromatosis type I are associated with 10% of PCC tumor. Hereditary cases are usually benign; however, approximately 5% to 26% of PCC (8) and 15% to 35% of PGL are malignant in nature (2).
Malignant PCC are even rarer and very difficult to diagnose microscopically. Malignancy is diagnosed when there is presence of local invasion or metastatic disease to bones, lymph nodes, liver, lung, and brain (5). We would like to report a case of malignant PCC treated with palliative radiotherapy and chemotherapy.
Case presentation
The patient is a 53-year-old male Taiwanese patient married with 2 children and works as a construction worker for >20 years. The patient smokes 1PPD × 25 years; he has quitted alcohol drinking and betel nut for >10 years. The patient has been under diabetes mellitus type 2 and gout medication control for >15 years and denies any family history of cancer.
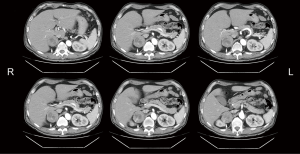
The patient suffered from lower back pain with radiation to both lower limbs was noted for years. He was treated with analgesic agent at local clinic for years, until the back pain became progressive and unbearable for 1 month. He then visited local community hospital for help. The patient was then transferred to our institution for further treatment. The patient’s vital sign on admission at 2016/7/29 showed a BP of 118/81, HR of 93/min, RR of 18/min, BT was 38 °C, pain score was 5–6, Glasgow coma scale was E4M6V5. The patient’s BP showed a paroxysmal pattern during admission, ranging from 87/53 to 226/142. Abdominal CT scan on 2016/7/26 revealed a 5.6×5.3×6.9 cm3 right adrenal heterogeneous mass (Figure 1). No metastatic regional lymph nodes were noted. Tc-99m whole body bone scan on 2016/8/15 was negative for bone metastasis.
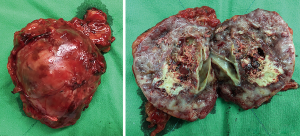
CT-guided biopsy of the right adrenal gland mass lesion on 2016/08/03 confirmed a pathologic diagnosis of PCC. Robot-assisted adrenalectomy of the right adrenal gland was performed on 2016/8/31. The right adrenal gland tumor was 7.3×6×4.5 cm3 in dimension, weights 100 g, partially encapsulated, brownish yellow in color and soft in consistency. The cut surface of the right adrenal tumor showed extensive necrosis and hemorrhage (Figure 2), there was still residual tumor in tumor bed area (R1 resection).
Microscopic sections showed discrete tumor cells nests in alveolar, trabecular and zellballen patterns. The tumor cells were characterized by oncocytic and basophilic cytoplasm, with abundant granular cytoplasm, polar nuclei, and small nucleoli. Focal spindle tumor cells showed anaplastic cells differentiation. Focal hemorrhages, necrosis, mitosis, capsular and vascular invasion were noted.
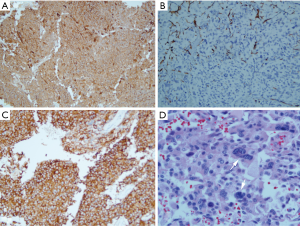
Histological sections stain positively for chromogranin A (100%, +++), synaptophysin (100%, +++), Ki-67 (40%), S100 stain was positive for sustentacular cells, the tumor stained negatively for TTF-1, RCC, CK7, CK20 and melan-A (Figure 3).
The patient remained hypertensive and hyperglycemic after surgery. Humulin insulin, oral glimepiride and metformin were given to control hyperglycemia. The final diagnosis was malignant PCC with vascular and capsular invasion. The patient’s staging is pT3N0M0, stage III (7th edition AJCC staging system). The patient’s overall PCC of the adrenal gland scales score (PASS) was 8 due to presence of capsular invasion [1], vascular invasion [1], atypical mitotic figures [2], high cellularity [2], and focal or confluent tumor necrosis [2].
The patient’s 24 h urine vanillylmandelic acid (VMA; normal range, 1–7.5 mg/24 h urine) dropped from 25.6 mg before surgery to 10 mg after tumor excision on 2016/8/31.
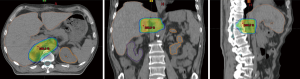
The patient received postoperative sliding window intensity modulated radiotherapy (IMRT) 3 weeks after R1 resection with a Varian 21EX linear accelerator 10 MV photon beam. Based on the preoperative right adrenal gland tumor volume and location, the clinical target volume (CTV) included the right adrenal fossa, paraaortic lymph nodes, medial aspect of the upper pole of the right kidney, right crura of the diaphragm, right perirenal fat tissue, right perinephric fascia, lateral wall of inferior vena cava and the posterior surface of segment 6 of the liver. The patient was treated in a supine position and immobilized with a vacuum bag to improve the reproducibility of daily treatments. A 2D kV orthogonal imaging was done weekly to verify patient setup during radiotherapy, using a surgical clip as fiducial marker.
Radiation was given to a total dose of 50 Gy in 25 fractions in 5 weeks, the planning target volume (PTV) was determined as the CTV plus a 5 mm margin, the 95% of PTV volume was covered by the 95% of prescribed dose (Figure 4). The mean doses to organs at risk (OARs) such as the liver (13 Gy), right kidney (6 Gy), left kidney (3 Gy), spinal cord (38 Gy) and stomach (14 Gy) were within normal tissue dose constraints. The patient tolerated the whole course of radiotherapy without incurring any grade 3 or grade 4 toxicities. Lysodren (Mitotane) was given to the patient after radiotherapy as an adjuvant oral adrenal cytotoxic agent for PCC, at 2 g daily.
Unfortunately, the patient later developed bacteremia and low-grade fever on 2017/05/06; sputum culture revealed methicillin resistant Staphylococcus aureus (MRSA) infection, the patient was intubated on 2017/05/14 for severe dyspnea caused by pneumonia of both lungs. The patient was followed up regularly on an out-patient basis for medications; no tumor recurrence was noted based on ultrasound study. Unfortunately, the patient went into septic shock and expired due to cardiac arrest 10 months after diagnosis of malignant PCC.
Discussion
PCC usually occurs between the third and fifth decades of life, of which 0.02–0.5% of PCC was diagnosed during a routine hypertensive workup (1). Most PCC are sporadic tumors, while 30–35% were familial benign PCC (7,9). Our patient is a sporadic case of PCC as the he does not have any family history of PCC; workup did not reveal any other neoplasia of the parathyroid, thyroid gland, pancreatic gland, renal cell carcinomas or neurofibromatosis.
Pathologic diagnosis of PCC can be made when the tumor exhibits well-circumscribed masses showing the characteristic Zellballen pattern under hematoxylin and eosin stain; these are nests of polygonal or rounded cells separated by peripheral capillaries (1,10). PCC cells should stain positive for chromogranin A and synaptophysin, as seen in Figure 3 (11). Another diagnostic criterion is the presence of sustentacular cells, stained positively with S100; these cells are seen at the periphery of Zellballen, or interspersed between tumor cells and functions as stem cells or glial-like supporting cells (10). A negative melan-A immunostaining rules out adrenal cortical adenoma (11), as was seen in our case.
The Ki-67 nuclear antigen represents a molecular marker for the proliferation potential of tumor cells. A Ki-67 index >3% connotes malignant potential (12). Our patient’s Ki-67 index was 40%, indicating a tumor with a highly malignant behavior.
PCC tumors showed high signal intensity on T2-weighted magnetic resonance imaging (MRI) study (1). MRI showed a higher accuracy (sensitivity 90–100% and specificity 50–100%) in detecting adrenal or extra-adrenal tumors, while CT scan has a sensitivity of 77–98% and specificity of 29–92% (12).
Functional imaging with 131I or 123I-metaiodobenzylguanidine (MIBG) scintigraphy can also be used to evaluate PCC and PGL since MIBG has chemical similarities to norepinephrine and is concentrated in chromaffin tissue (12), but it is not available in our hospital. A careful review of the patient’s history and physical examination provided us with the initial clinical impression of PCC, whole abdomen CT scan was able to provide for a diagnostic confirmation in this patient.
Hypertension is the most common feature of PCC and PGL: it can be continuous, intermittent, and often paroxysmal in nature, patient usually exhibited a triad of severe headache, palpitations, and diaphoresis when in hypertensive crisis (12). Our patient showed a long continuous history of hypertension, with occasional hypertensive crisis that was controlled by antihypertensive medication.
A diagnostic criterion of malignant PCC is the presence of tumor invasion of adjacent structures or metastasis to sites where chromaffin tissue is normally absent such as lymph nodes, liver, lungs, and bones. Approximately 3% to 36% of PCC are malignant (9,12,13). Aggressive tumor growth is characterized by histologic features of spindle morphology, increased mitotic rate, and invasion of the organ’s capsule. (1), or tumor size ≥5 cm (8,13,14).
The PASS score based on the degree of tumor invasion, histologic growth patterns, cytologic features, or mitotic activity is used to distinguish a benign from a malignant PCC. A PASS score of <4 suggest benign lesion, a PASS score from 4 to 6 suggests an intermediate risk, while malignant PCC has a score >6 (11,12). Our patient’s PASS is 8 out of a maximum score of 20, making it a malignant PCC.
Complete surgical resection is the only curative treatment option for benign PCC and its associated hypertension. For malignant PCC, surgical debulking of the tumor and resection of distant metastases whenever possible, although not curative, is regarded as the mainstay of therapy (12).
131I-MIBG was used to treat malignant, non resectable or metastatic PCC. A single or fractionated dose ranging from 200–1,400 mCi have been used. About 60% of metastatic sites are 131I-MIBG avid and shows better responses in soft-tissue metastases than in bone metastases. However, treatment with 131I-MIBG is not curative in most patients (12). Response rate ranged from 0% to 87% (15). Unfortunately, 131I-MIBG therapy was not been available for therapeutic purpose in Taiwan.
Adjuvant external beam radiotherapy was recommended after surgical debulking of PCC, especially in patients with incomplete or R1 resection (12,16).
Siddiqui et al. (17) irradiated a recurrent PCC mass with 60Co for 30.6 Gy in 18 fractions, repeat CT scan showed shrinkage of the mass. Palliative radiotherapy for bone pain relief was also given. Chemotherapy with the cyclophosphamide, dacarbazine and vincristine was given at 3 weeks interval for 2 courses. The patient died of metastasis 12 months after diagnosis. The authors concluded that radiotherapy and chemotherapy showed good therapeutic response in malignant PCC.
Yu et al. (15) was able to reverse cranial nerve deficit due to PCC metastatic tumor in the parasellar region by giving 25 Gy, the patient suffered a local recurrence again 2 years later, and was again treated with 20 Gy, achieving prompt tumor response, the patient’s hepatic metastasis was controlled for >2 year by giving 32.4 Gy. They recommended a dose of 40–50 Gy to achieve a more long-lasting tumor control for inoperable tumor.
You et al. (18) reported a 23-year-old male patient presenting with a primary malignant PCC of the urinary bladder invading the prostate. The patient refused radical surgery and was treated with external radiotherapy for a total of 66 Gy in 30 fractions. The patient’s MRI revealed reduced tumor size, blood pressure became normal and was still alive 1.5 years after radiotherapy. You et al concluded that radiotherapy was an effective treatment to a certain extent for malignant PCC.
Vogel et al. (9) treated 24 malignant PCC with radiotherapy to tumors located in the abdomen (n=3), central nervous system (n=4), and bone (n=40). The radiotherapy mean dose was 31.8 Gy (25–55 Gy), or 21.9 Gy in 1.36 Gy fractions with fractionated stereotactic radiosurgery (FSRT). Overall local control was 86.7%, symptomatic control was 81.1%. The average survival was 52.4 months. The study showed better symptomatic responses, local control in patients who received higher doses of radiation. Vogel et al. (9) concluded that radiotherapy plays a significant role in the control of malignant PCC and outcomes may improve with higher doses of radiation over 40 Gy.
The overall 5-year survival for benign and malignant PCC was 90% to 96% and 34 to 72% respectively; poorer survival is associated with the presence of pulmonary metastases. (1,9). Patients with liver or lung metastases have a worse prognosis (<5 years) than patients with isolated bone lesions (1,12). Cox multivariate analysis of 287 malignant PCC between 1988 and 2009 by Goffredo et al. (14) reveals that factors associated with higher overall mortality included age 61–76 years, tumor size ≥5 cm, distant metastases, and unresectable disease necessitating palliative radiotherapy.
Conclusions
Malignant PCC is a rare and unusual neuroendocrine tumor seen in the clinic. Although both benign and malignant PCC have the same radiographic findings, the presence of multiple tumors and a PASS score of >6 indicate a malignant tumor. IMRT should be considered as an effective postoperative adjuvant therapy for malignant PCC, more case study is needed to clarify the role of radiotherapy for this type of tumor.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2019.08.02). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal. The author confirms that the images in this report are from the patient’s medical record and is not derived from any previously published image.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Johnson MH, Cavallo JA, Figenshau RS. Malignant and metastatic pheochromocytoma: case report and review of the literature. Urol Case Rep 2014;4:139-41. [Crossref] [PubMed]
- Harari A, Inabnet WB 3rd. Malignant pheochromocytoma: a review. Am J Surg 2011;201:700-8. [Crossref] [PubMed]
- Arnas-Leon C, Sánchez V, Santana Suárez AD, et al. Complete remission in metastatic pheochromocytoma Treated with Extensive Surgery. Cureus 2016;8:e447 [PubMed]
- Andersen KF, Altaf R, Krarup-Hansen A, et al. Malignant pheochromocytomas and paragangliomas - the importance of a multidisciplinary approach. Cancer Treat Rev 2011;37:111-9. [Crossref] [PubMed]
- Hamidi O, Young WF Jr, Iñiguez-Ariza NM, et al. Malignant pheochromocytoma and paraganglioma: 272 patients over 55 years. J Clin Endocrinol Metab 2017;102:3296-305. [Crossref] [PubMed]
- Fränkel F. Classics in oncology. A case of bilateral completely latent adrenal tumor and concurrent nephritis with changes in the circulatory system and retinitis. CA Cancer J Clin 1984;34:93-106. [Crossref] [PubMed]
- Young WF. Endocrine hypertension. In: Melmed S, Polonsky K, Larsen PR, et al. editors. Williams Textbook of Endocrinology. 12th edition. Elsevier Saunders, 2011:545-77.
- Feng F, Zhu Y, Wang X, et al. Predictive factors for malignant pheochromocytoma: analysis of 136 patients. J Urol 2011;185:1583-90. [Crossref] [PubMed]
- Vogel J, Atanacio AS, Prodanov T, et al. External beam radiation therapy in treatment of malignant pheochromocytoma and paraganglioma. Front Oncol 2014;4:166. [Crossref] [PubMed]
- Tischler AS, deKrijger RR. 15 YEARS OF PARAGANGLIOMA: Pathology of pheochromocytoma and paraganglioma. Endocr Relat Cancer 2015;22:T123-33. [Crossref] [PubMed]
- Lloyd RV. Adrenal cortical tumors, pheochromocytomas and paragangliomas. Mod Pathol 2011;24:S58-65. [Crossref] [PubMed]
- Parenti G, Zampetti B, Rapizzi E, et al. Updated and new perspectives on diagnosis, prognosis, and therapy of malignant pheochromocytoma/paraganglioma. J Oncol 2012;2012:872713 [Crossref] [PubMed]
- Huang KH, Chung SD, Chen SC, et al. Clinical and pathological data of 10 malignant pheochromocytomas: long-term follow up in a single institute. Int J Urol 2007;14:181-5. [Crossref] [PubMed]
- Goffredo P, Sosa JA, Roman SA. Malignant pheochromocytoma and paraganglioma: a population level analysis of long- term survival over two decades. J Surg Oncol 2013;107:659-64. [Crossref] [PubMed]
- Yu L, Fleckman AM, Chadha M, et al. Radiation therapy of metastatic pheochromocytoma: case report and review of the literature. Am J Clin Oncol 1996;19:389-93. [Crossref] [PubMed]
- Berruti A, Baudin E, Gelderblom H, et al. Adrenal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:vii131-38. [PubMed]
- Siddiqui MZ, Von Eyben FE, Spanos G. High-voltage irradiation and combination chemotherapy for malignant pheochromocytoma. Cancer 1988;62:686-90. [Crossref] [PubMed]
- You D, Ren R, Chen E, et al. Radiotherapy for urinary bladder pheochromocytoma with invasion of the prostate: a case report and literature review. Mol Clin Oncol 2016;4:1060-2. [Crossref] [PubMed]
Cite this article as: Yeh CY. Radiotherapy of malignant pheochromocytoma—a case report. Ther Radiol Oncol 2019;3:31.