Brachial plexus doses in locoregional radiotherapy for breast cancer
Introduction
Radiation-induced neuropathy involving the brachial plexus has been a cause of concern over many decades despite the low overall reported incidence, considering the potentially irreversible nature of injury and its long-term implications on functional quality of life in survivors. It has been reported variously in axillary and supraclavicular irradiation to doses of 30–70 gray (Gy) in mantle field irradiation for lymphomas, locoregional radiotherapy (LRRT) for breast cancer, apical irradiation in lung cancers as well as neck irradiation for head and neck malignancies (1-4). Consensus recommendations on brachial plexus dose tolerance by Emami et al. considered the brachial plexus and cauda equina together and suggested a value for a 5% risk at 5 years of 62, 61, and 60 Gy and a value for a 50% risk at 5 years of 77, 76, and 75 Gy for one-third, two-thirds, and the whole organ, respectively (5). Consequently, many Radiation Therapy Oncology Group (RTOG) protocols such as RTOG 0617 and RTOG 0619 have placed a maximum dose constraint of 60–66 Gy, delivered at 2 Gy per fraction for brachial plexus (6,7). In recent times, availability of stereotactic techniques have renewed interest in hypofractionation for treatment of primary or recurrent tumors, exposing the late reacting tissues in the vicinity including brachial plexus to even greater risk, and the need to identify and define tolerance doses for various fractionation schedules.
The practice of locoregional irradiation is rapidly giving way to omission of axillary radiotherapy in the setting of adequate axillary clearance due to concerns of lymphedema and brachial plexus neuropathy (BPN), but axillary radiation with posterior axillary boost is still being practiced for locally advanced breast cancer (LABC) at several institutions, including ours, in the absence of convincing evidence against its omission, especially in the presence of heavy axillary nodal involvement.
The brachial plexus contouring guidelines have been described and validated by RTOG for head and neck cancers (8). However, the usual treatment position for breast cancer radiotherapy with breast board incline and shoulder abduction leads to a considerable distortion of normal anatomy. Kong et al., have described the contouring guidelines for normal structures including brachial plexus, relevant for use in sterotactic irradiation in thoracic tumors (9). Recently, cadaver-based anatomic validation of computed tomography (CT) and magnetic resonance imaging (MRI) based brachial plexus delineation has been described and the authors suggested a 4.7 mm expansion around this contour for including anatomic variations to achieve 100% coverage of brachial plexus (10). This approach, however, has been criticised since it might compromise useful dose delivery necessary for control of gross disease in this region (11).
We intended to study the feasibility of identifying the brachial plexus in the planning CT scans for breast cancer radiotherapy, and to retrospectively evaluate the brachial plexus doses in patients who had received supraclavicular and axillary irradiation.
Methods
Patients
We retrieved the charts of 10 patients with LABC, who had completed post-mastectomy radiation therapy to chest wall, supraclavicular fossa and axilla at our department in the period April 2010 to July 2010. Clinical and demographic details of these patients were noted. Non-contrast planning CT sets (3 mm slice thickness) of these patients were retrieved from the planning system (CMS Xio). The anatomy of normal brachial plexus was studied using anatomy and radiology texts and publications. RTOG guidelines, American Society of Radiation Oncology Continuing Medical Education (ASTRO CME) and anatomy texts were consulted for brachial plexus contouring (8,12-14).
Contouring
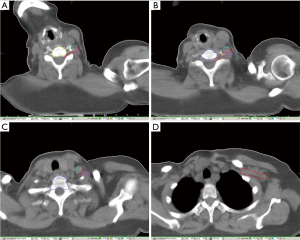
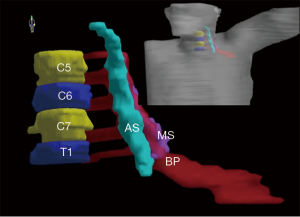
Vertebral bodies C5–7 and T1 were identified on planning CT sets. Ipsilateral anterior and middle scalene muscles (MS) were identified and delineated. In certain slices near C5–6 vertebrae where it was difficult to distinguish the middle from posterior scalene muscle due to neck rotation, both middle and posterior scalene were contoured as one structure. Subsequently the five nerve roots of brachial plexus (uppermost arising just above C5 and lowermost below T1) originating in the intervertebral space and passing laterally between anterior and middle scalene were identified and contoured. The subclavian vessels were identified as far laterally as possible till continuation with axillary vessels. The trunks of brachial plexus are described as lying posterior and superior to subclavian vessels. However, in the non-contrast scans, it was difficult to distinguish the exact planes in this location and the entire neurovascular bundle was contoured as surrogate for brachial plexus as described in RTOG guidelines. Figures 1,2 show the axial contours and 3D reconstruction of brachial plexus delineation.
Treatment protocol
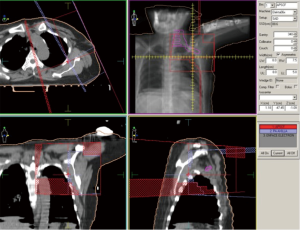
The dose to the entire locoregional area was 50 Gy in 25 fractions, prescribed to 95% isodose, delivered over 5 weeks. Chest wall was irradiated using 6–10 Mega-electronVolt (MeV) electrons, prescribed at 80–100% isodose, depending on chest wall thickness. Axilla and supraclavicular region were treated using photon fields matched with the electron field used for chest wall radiotherapy. An anterior photon field of 6 megavolts (MVs), with cord shielding from beginning, with a prescription of 40 Gy in 20 fractions was opposed by a posterior axillary field (6 MVs) delivering additional dose of 10 Gy in 5 fractions. Figure 3 shows the beam arrangement for a representative plan.
Following delineation of brachial plexus on all 10 CT sets, the treatment plans were recalculated without changing any of the prescription parameters such as reference point or prescription isodose. The global dose maximum of individual plans, and the maximum and median brachial plexus doses in these plans, were recorded.
Results
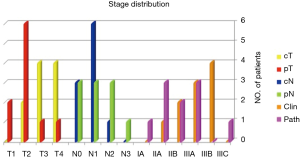
Our study population consisted of 10 women with median age 47 years (range, 40–65 years), with LABC. Figure 4 shows the TNM stage distribution (as per AJCC 7) of these patients. All of them had undergone modified radical mastectomy followed by 6–8 cycles of adjuvant chemotherapy before being planned for locoregional radiation therapy. Four had right-sided disease while six had left-sided tumors.
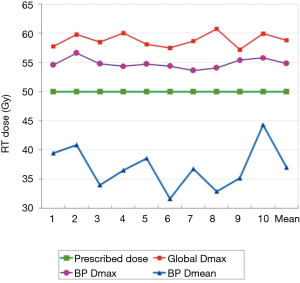
A total dose of 50 Gy in 25 fractions was planned, prescription isodose being 95%. The global dose maximum of the respective plans ranged from 57.19 to 60.75 Gy (mean 58.83 Gy).
Mean ipsilateral brachial plexus volume was 13.8 cc (range, 12.1–16.1 cc). For a prescription of 50 Gy, the maximum brachial plexus dose ranged from 53.64 to 56.61 Gy (median 54.65 Gy) and the mean dose ranged from 31.61 to 44.27 Gy (median 36.62 Gy). Maximum brachial plexus dose was always higher than the prescription dose. Mean brachial plexus volume receiving a dose higher than 50 Gy was 27.81% (median 22.01%, range, 13.01–51.80%). Figure 5 shows the brachial plexus doses compared to prescription doses in each of the 10 plans.
Discussion
BPN is a rare but serious side effect of breast cancer radiation therapy. A Royal College of Radiologists’ report noted 48 patients with BPN following radiotherapy for breast cancer in the period 1980–1993. Patients were treated in a semisupine position for primary site while the position was changed to supine for nodal irradiation. This policy may have contributed to field overlaps and overdoses to brachial plexus near the junctional region. This report showed a direct correlation between the incidence of radiation-induced BPN and movement of the patient movement between treatment fields. All 12 (100%) patients who had major intrafractional positional changes developed BPN while the incidence was 15% for those with no change in position. The change from cobalt-60 to linear accelerator-based radiotherapy led to standardization of immobilization in the mid-1980s. Of the 48 reported cases, 41 developed BPN in first 7 years, while there were only 7 other cases in the remaining period, reflecting changes in practice. The report suggested that nodal irradiation should be individualized and protocol-based, and that the patient position should remain unchanged for both breast and supraclavicular fossa-axilla irradiation (15,16).
Although the complication of BPN has been most often described for breast cancer in literature, studies that have formally evaluated brachial plexus doses in breast cancer patients are scarce. Conventional doses of 50–54 Gy in 2 Gy fractions used for breast cancer irradiation are well below the described tolerance dose of brachial plexus. The estimated equivalent dose at 3 cm depth (for brachial plexus) can be as high as 61 Gy for a “50 Gy in 25 fractions” prescription with an anterior and posterior axillary field, as described in the START trial protocol (15). A Danish study reported by Olsen et al reported the incidence of BPN as 5% in women receiving axillary radiotherapy, the highest rates being in women with absorbed doses at brachial plexus (surrogate at 3 cm depth) in 53–70 Gy range (17). A retrospective analysis of brachial plexus doses with anteroposterior supraclavicular irradiation with cobalt-60 units using 3 Gy per fraction suggested a dose equivalent of 59.8 Gy to brachial plexus (surrogate at 3 cm depth), with a 5-year incidence of 3.9%, with an annual incidence of 2.9% for grade ≥1 lesions and 0.8% for grade ≥3 lesions. The risk remained constant with time (18). A recent dosimetric study has also described mean brachial plexus dose maxima of 52.22 Gy with addition of supraclavicular field but no axillary irradiation (19). Our dosimetric study corroborates with these findings of high dose regions within the brachial plexus contoured volumes.
It is well-known that BPN may result not only from the nerve damage which has a relatively higher threshold as noted in the review by Emami et al., but also from progressive ischemic changes and fibrosis around nerve trunks which may depend on the total dose as well as dose per fraction. The incidence of BPN may be 5–6 times higher with doses of 3 Gy or above compared with treatment schedules delivering less than 2 Gy per fraction (20-22). The 10-year follow up of START A & B trials challenges these findings, with no significant increase in incidence of BPN in patients randomized to 40 Gy in 15 fractions, 39 Gy in 13 fractions or 41.6 Gy in 13 fractions (23). A literature review by Gałecki et al., showed that the risk of brachial plexus injury varies from 1.7% to 73% in the dose range of 43.5 to 60 Gy at 2.2–4.58 Gy per fraction. The risk was under 1% with doses less than 40 Gy delivered at 2.5 Gy per fraction or lower. With increasing biologically effective doses above 55 Gy, there is rapid increase in risk. Additional factors contributing to increased risk include surgical axillary manipulation and chemotherapy (24).
The above literature findings affirm that even in the therapeutic dose range for adjuvant irradiation in breast cancer, the risk of BPN is significant and depends on a host of factors including total dose, dose per fraction, treatment technique in addition to factors such as patient anatomy, surgical manipulation and systemic therapy. This risk is even higher with uncertain distribution of hot spots in conformal or intensity modulated radiotherapy, and remains to a great extent even with omission of axillary radiotherapy in recent protocols. Patients with breast conservation for upper outer quadrant disease, who receive additional tumor bed boost, may get additional dose contribution to brachial plexus. Considering this risk, it is imperative that modern day radiotherapy accounts for all these factors. Thus, the identification of brachial plexus, evaluation of dose delivered and if necessary, plan modifications to keep this dose within the risk-free range have become necessary, even if the apparent prescription dose lies within the tolerance range of brachial plexus.
To summarise, brachial plexus contouring in breast cancer planning CT scans is feasible, with some uncertainty in regions of altered anatomy (C5–6, shoulder). In our study, the maximum brachial plexus doses always exceeded prescribed dose of 50 Gy, and although lower than the allowed tolerance dose of 60–66 Gy, should be evaluated to reduce the risk of adverse events.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2019.08.03). The authors have no conflicts of interest to declare. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This was a dosimetric study with no implications for patients’ privacy or safety. No formal ethics approval was needed.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wadd NJ, Lucraft HH. Brachial plexus neuropathy following mantle radiotherapy. Clin Oncol (R Coll Radiol) 1998;10:399-400. [Crossref] [PubMed]
- Johansson S, Svensson H, Larsson LG, et al. Brachial plexopathy after postoperative radiotherapy of breast cancer patients - a long-term follow-up. Acta Oncol 2000;39:373-82. [Crossref] [PubMed]
- Forquer JA, Fakiris AJ, Timmerman RD, et al. Brachial plexopathy from stereotactic body radiotherapy in early-stage NSCLC: dose-limiting toxicity in apical tumor sites. Radiother Oncol 2009;93:408-13. [Crossref] [PubMed]
- Chen AM, Hall WH, Li BQ, et al. Intensity-modulated radiotherapy increases dose to the brachial plexus compared with conventional radiotherapy for head and neck cancer. Br J Radiol 2011;84:58-63. [Crossref] [PubMed]
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109-22. [Crossref] [PubMed]
- RTOG 0617. A randomized phase III comparison of standard- dose (60 Gy) versus high dose (74 Gy) conformal radiotherapy with concurrent and consolidation carboplatin/paclitaxel +/- cetuximab (IND #103444) in patients with stage IIIA/IIIB non-small cell lung cancer. [updated 2011 Nov 06; cited 2019 Aug 26]. Available online: https://www.rtog.org/LinkClick.aspx?fileticket=QU8LgXgAimk%3D&tabid=290
- RTOG 0619. A randomized phase II trial of chemoradiotherapy versus chemoradiotherapy and vandetanib for high-risk postoperative advanced squamous cell carcinoma of the head and neck. [updated 2010 Feb 25; cited 2019 Aug 26]. Available online: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0619
- Hall WH, Guiou M, Lee NY, et al. Development and validation of a standardized method for contouring the brachial plexus: preliminary dosimetric analysis among patients treated with IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2008;72:1362-7. [Crossref] [PubMed]
- Kong FM, Ritter T, Quint DJ, et al. Consideration of dose limits for organs at risk of thoracic radiotherapy: atlas for lung, proximal bronchial tree, esophagus, spinal cord, ribs, and brachial plexus. Int J Radiat Oncol Biol Phys 2011;81:1442-57. [Crossref] [PubMed]
- Van de Velde J, Audenaert E, Speleers B, et al. An anatomically validated brachial plexus contouring method for intensity modulated radiation therapy planning. Int J Radiat Oncol Biol Phys 2013;87:802-8. [Crossref] [PubMed]
- Basu T, Kataria T, Gupta D, et al. An anatomically validated brachial plexus contouring method for intensity modulated radiation therapy planning. In regard to Van de Velde et al. Int J Radiat Oncol Biol Phys 2014;89:224. [Crossref] [PubMed]
- van Es HW, Bollen TL, van Heesewijk HP. MRI of the brachial plexus: a pictorial review. Eur J Radiol 2010;74:391-402. [Crossref] [PubMed]
- Agur AMR, Dalley AF. Grant’s atlas of anatomy. 12th edition. Lippincott Williams & Wilkins, 2009:507-10.
- Truong MT, Nadgir RN, Hirsch AE, et al. Brachial plexus contouring with CT and MR imaging in radiation therapy planning for head and neck cancer. Radiographics 2010;30:1095-103. [Crossref] [PubMed]
- Dobbs HJ. Radiation therapy for breast cancer at the millennium. Radiother Oncol 2000;54:191-200. [Crossref] [PubMed]
- Spittle MF. Brachial plexus neuropathy after radiotherapy for breast cancer. BMJ 1995;311:1516-7. [Crossref] [PubMed]
- Olsen NK, Pfeiffer P, Johannsen L, et al. Radiation induced brachial plexopathy: Neurological follow up in 161 recurrence-free breast cancer patients. Int J Radiat Oncol Biol Phys 1993;26:43-9. [Crossref] [PubMed]
- Bajrovic A, Rades D, Fehlauer F, et al. Is there a life-long risk of brachial plexopathy after radiotherapy of supraclavicular lymph nodes in breast cancer patients? Radiother Oncol 2004;71:297-301. [Crossref] [PubMed]
- Stanic S, Mathai M, Mayadev JS, et al. Addition of a third field significantly increases dose to the brachial plexus for patients undergoing tangential whole-breast therapy after lumpectomy. Med Dosim 2012;37:127-30. [Crossref] [PubMed]
- Gosk J, Rutowski R, Reichert P, et al. Radiation-induced brachial plexus neuropathy - aetiopathogenesis, risk factors, differential diagnostics, symptoms and treatment. Folia Neuropathol 2007;45:26-30. [PubMed]
- Stoll BA, Andrews JT. Radiation-induced peripheral neuropathy. BMJ 1966;1:834-7. [Crossref] [PubMed]
- Powell S, Cooke J, Parsons C. Radiation-induced brachial plexus injury: follow-up of two different fractionation schedules. Radiother Oncol 1990;18:213-20. [Crossref] [PubMed]
- Haviland JS, Owen JR, Dewar JASTART Trialists' Group, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013;14:1086-94. [Crossref] [PubMed]
- Gałecki J, Hicer-Grzenkowicz J, Grudzień-Kowalska M, et al. Radiation-induced brachial plexopathy and hypofractionated regimens in adjuvant irradiation of patients with breast cancer--a review. Acta Oncol 2006;45:280-4. [Crossref] [PubMed]
Cite this article as: Goyal S, Menon D, Puzhakkal N, Makuny D. Brachial plexus doses in locoregional radiotherapy for breast cancer. Ther Radiol Oncol 2019;3:30.