
Chemo-radiotherapy for olfactory neuroblastoma: cases report and literature review
Introduction
Olfactory neuroblastoma was first described by Berger and Richard in 1924. It is also named as esthesioneuroblastoma (1). It has been characterized as a rare malignant neuroectodermal neoplasm of the sinonasal cavity, which typically occurs in superior nasal cavity medial to the middle turbinate and represents about 3% of all sinonasal malignancies (2). The precise location and histogenesis of olfactory neuroblastoma are not clearly known. The most accepted site of origin is the basal neural cells of the olfactory mucosae and then arises in the superior portion of the nasal vault (3). The clinical behavior of olfactory neuroblastoma ranges widely from relatively indolent to both locally aggressive and metastatic. Most of the symptoms are related to the anatomic structures affected by mass effect or local invasion (4).
It is challenging for characterization and treatment due to its rare incidence. There are three modalities used to treat olfactory neuroblastoma: surgery, external beam radiation, and chemotherapy. The optimal treatment regimens are still under investigation. Often, a combination of these three modalities is used, namely surgical resection with postoperative irradiation, for all but the smallest tumors (5,6). Chemotherapy has a role for more advanced cases, but the utility of chemotherapeutic agents is not well-defined. For unresectable local disease, chemo-radiation is an approach but lack of strong evidence for the combination of radiation dose and chemotherapy regimen.
Case presentation
Two patients were initially diagnosed as stage IVA olfactory neuroblastoma via tumor biopsy and staged properly by serial images evaluation including head and neck magnetic resonance imaging (MRI), chest X-ray, abdominal sonography and bone scan. Patient was treated with chemo-radiotherapy after diagnosis. After completion of chemo-radiotherapy, they were regularly followed up at out-patient clinic. The treatment toxicity and complication were evaluated by physical examination and history-taking. The tumor response was evaluated by head and neck MRI.
Case 1 was a 48-year-old female patient, cT4aN2cM0, stage IVA, treated with radiotherapy 70 Gy in 35 fractions (2 Gy per fraction) to clinical target volume of high risk area (CTV-H) (right nasal mass + right ethmoid sinus mass + subfrontal mass + bilateral upper 2/3 neck lymphadenopathy), 59.5 Gy in 35 fractions to clinical target volume of low risk area (CTV-L) (CTV-H with 0.3–1 cm margin added + sphenoid sinus + ethmoid sinus + retropharyngeal lymph nodes + bilateral neck lymph nodes), and concurrent intravenous Cisplatin 30 mg/m2 for one course. The image comparison between pre- and post- treatment was displayed on Figure 1. The upper row of images was taken on 8th February in 2010, 3 weeks after completion of treatment. The MRI showed a large lobulated soft tissue mass lesion, about 3.1 cm ×4.2 cm ×4.9 cm in size, which was involving right middle to superior nasal turbinates and walls, nasal septum. It also invaded into ethmoid sinuses, cribriform plate and olfactory groove. The tumor showed partial remission after serial image follow-up. The lowest row of images was taken on 11th September in 2018. The tumor was measured 2.4 cm ×3.46 cm ×0.94 cm in size, which is in the anterior cranial fossa base in the epidural olfactory groove and with destruction of cribriform plate and extension through the perpendibular plate ethmoid bone. The follow-up MRI scan of the head and neck showed no interval change since 12th May in 2016 regarding the size of the residual tumor in the olfactory groove in the intracranial epidural space, as well as in the cribriform and perpendicular plates of the ethmoid bone. She was recurrence-free for 9 years.
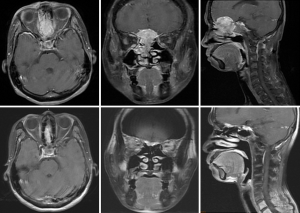
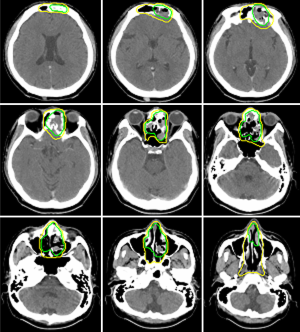
Case 2 was a 38-year-old male patient, cT4N1M0, stage IVA, treated by induction chemotherapy with intravenous Etoposide + Carboplatin for 4 courses from 21st March to 30th May in 2011 and followed by radiotherapy 70 Gy in 35 fractions (2 Gy per fraction) to CTV-H (ethmoid sinus mass & bilateral level IIa lymphadenopathy) and 59.5 Gy in 35 fractions to CTV-L (CTV-H with margin added + frontal sinus + nasopharynx + nasal cavity + bilateral retropharyngeal lymph nodes + level II-IV lymph nodes) from 21st June to 5th August in 2011. Contouring of CTV-H and CTV-L was shown on Figure 2. The image comparison between pre- and post- treatment was displayed on Figure 3. The upper row of MRI images was taken on 18th Feb in 2011. There was an irregular mass (4 cm ×4.7 cm ×5 cm in size) within the ethmoid sinuses with bone destruction and extending into the left frontal sinus. The lower row of MRI images was taken on 2nd April in 2016. No evident tumor was identified in the nasal cavity. Comparison between these two series of images, the tumor showed complete remission. From the latest follow-up date on 25th February in 2019, he was disease-free without recurrence for 8 years.
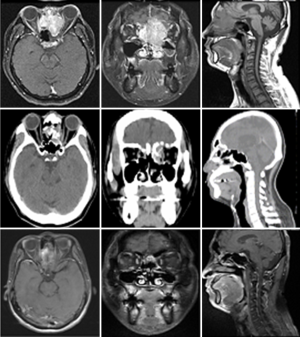
These two cases demonstrated that definitive chemo-radiotherapy would be an alternative treatment option for olfactory neuroblastoma. They received definitive chemo-radiotherapy without any acute or chronic toxicity. Regular head and neck MRI were performed for follow-up. The tumor was well controlled without recurrence.
Discussion
The optimal treatment strategy for olfactory neuroblastoma is difficult to determine, because of its rarity and anatomic location. Single-modality treatment, usually surgical resection, is suggested for small local tumor without metastasis (5,6). Multimodality combination is usually recommended for advanced stage cases. The treatment content has evolved over the years due to the advanced in radiation and surgery techniques. Surgical resection accompanied with post-operative radiotherapy showed well outcomes of overall survival and improving local control (6-8). However, it is still controversial to the use of chemotherapy alone, regarding the optimal choices of drug or indication.
Radiation plays an important role in treating olfactory neuroblastoma as the primary modality of treatment, as the preoperative setting or as the postoperative setting (9,10). Several studies have shown that operation with irradiation is superior to definitive radiotherapy (4,11). Radiation alone or chemo-radiotherapy is suggested when surgical is not an option due to medical contraindication or metastatic tumor status. Now intensity modulated radiation therapy (IMRT) is able to provide good sparing of optic nerve, brain and other normal structure. Total dose of 65–70 Gy under 1.8–2 Gy per fractions is most suggested (12).
The evidence supporting chemotherapy as the primary modality of treatment to olfactory neuroblastoma is limited. The role of chemotherapy is not clearly identified in the literature. It is generally applied in the setting of induction chemotherapy or concurrent chemo-radiation therapy. Cisplatin + Etoposide is the most accepted regimen for treating olfactory neuroblastoma (5,13,14). Chao et al. show good response to neoadjuvant chemotherapy with 4 cycles of Cyclophosphamide/Vincristine or Cisplatin/Etoposide (15). For patient with distant metastasis, systemic treatment is warranted. However, there is still no standard chemotherapy regimen for neither palliative nor curative chemotherapy.
Chemo-radiation is an approach for advanced tumor status. It may provide down-staging in pre-operative setting and improve the resectability for locally advanced olfactory neuroblastoma. Definitive chemo-radiation for olfactory neuroblastoma is rarely reported. In our study, we demonstrated definitive chemo-radiotherapy would be an alternative treatment option for olfactory neuroblastoma. The tumor was well controlled without recurrence for at least 8 years.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2019.07.01). JFC serve as an unpaid editorial board members of Therapeutic Radiology and Oncology from Oct 2017 to Sep 2019. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This research was approved by TMU-Joint Institutional Review Board (N201812027) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Berger L, Luc R, Richard D. L’esthesioneuroepitheliome Olfactif, Bulletin de l’Association de France d’étude. Cancer 1924;13:410-21.
- Faragalla H, Weinreb I. Olfactory neuroblastoma: a review and update. Adv Anat Pathol 2009;16:322-31. [Crossref] [PubMed]
- Dulguerov P, Allal AS, Calcaterra TC. Esthesioneuroblastoma: a meta-analysis and review. Lancet Oncol 2001;2:683-90. [Crossref] [PubMed]
- Ward PD, Heth JA, Thompson BG, et al. Esthesioneuroblastoma: and Outcomes of a Single Institution's Experience. Skull Base 2009;19:133-40. [Crossref] [PubMed]
- Saade RE, Hanna EY, Bell D. Prognosis and biology in esthesioneuroblastoma: the emerging role of Hyams grading system. Curr Oncol Rep 2015;17:423. [Crossref] [PubMed]
- Schwartz JS, Palmer JN, Adappa ND. Contemporary management of esthesioneuroblastoma. Curr Opin Otolaryngol Head Neck Surg 2016;24:63-9. [Crossref] [PubMed]
- Nishimura H, Ogino T, Kawashima M, et al. Proton-beam therapy for olfactory neuroblastoma. Int J Radiat Oncol Biol Phys 2007;68:758-62. [Crossref] [PubMed]
- Ow TJ, Bell D, Kupferman ME, Demonte F, Hanna EY. Esthesioneuroblastoma. Neurosurg Clin N Am 2013;24:51-65. [Crossref] [PubMed]
- Ozsahin M, Gruber G, Olszyk O, et al. Outcome and prognostic factors in olfactory neuroblastoma: a rare cancer network study. Int J Radiat Oncol Biol Phys 2010;78:992-7. [Crossref] [PubMed]
- Foote RL, Morita A, Ebersold MJ, et al. Esthesioneuroblastoma: the role of adjuvant radiation therapy. Int J Radiat Oncol Biol Phys 1993;27:835-42. [Crossref] [PubMed]
- Jethanamest D, Morris LG, Sikora AG, et al. Esthesioneuroblastoma: a population-based analysis of survival and prognostic factors. Arch Otolaryngol Head Neck Surg 2007;133:276-80. [Crossref] [PubMed]
- Suriano M, De Vincentiis M, Colli A, et al. Endoscopic treatment of esthesioneuroblastoma: a minimally invasive approach combined with radiation therapy. Otolaryngol Head Neck Surg 2007;136:104-7. [Crossref] [PubMed]
- Resto VA, Eisele DW, Forastiere A, et al. Esthesioneuroblastoma: the Johns Hopkins experience. Head Neck 2000;22:550-8. [Crossref] [PubMed]
- Bhattacharyya N, Thornton AF, Joseph MP, et al. Successful treatment of esthesioneuroblastoma and neuroendocrine carcinoma with combined chemotherapy and proton radiation. in 9 cases. Arch Otolaryngol Head Neck Surg 1997;123:34-40. [Crossref] [PubMed]
- Chao KS, Kaplan C, Simpson JR, et al. Esthesioneuroblastoma: the impact of treatment modality. Head Neck 2001;23:749-57. [Crossref] [PubMed]
Cite this article as: Wu JY, Chiou JF, Ting LL. Chemo-radiotherapy for olfactory neuroblastoma: cases report and literature review. Ther Radiol Oncol 2019;3:26.


