
Primary chemo-radiotherapy for breast cancer patients who refused surgical treatment: a case series
Introduction
Breast cancer (BC) is the most commonly diagnosed malignancy in women worldwide. The epidemiology is similar in Taiwan, where the incidence is 1 in 120 women and is rising (1). Mastectomy or BCS follow by adjuvant radiotherapy (RT) and systemic chemotherapy is the mainstay of treatment for localized disease. Patients with locally advanced stage (stage III and up) usually receive neoadjuvant chemotherapy followed by surgery and adjuvant RT (2). A minority of patients, usually with medical comorbidity or by personal decision, refuse any form of surgical treatment, which substantially affects life expectancy. In the 1970s, this group of patients was treated with RT alone, with distant metastasis as the major cause of mortality (3).
With the advance in treatment strategies, cancer prognosis has improved dramatically in the past 20 years. Chemotherapy acts as a radiosensitizer to improve local control (LC) and survival over the use of RT alone. Primary concurrent chemoradiotherapy (CCRT) is the first choice in treating nasopharyngeal (4), locally advanced cervical (5) and anal cancers (6). However, CCRT is not the main “player” in the treatment for BC. Several phase III studies exploring the effect of CCRT in early BCs reported good LC but increased cardiac and pulmonary toxicities (7). In locally advanced BC, adjuvant CCRT resulted in a better locoregional recurrence free survival rate compared to sequential CCRT (8). A study from Japan also reported a pathologically complete response rate of 36% in 108 stage I to IIIA patients who received neoadjuvant CCRT (9).
The role of definitive CCRT has been reserved for patients who are older or cannot tolerate surgical treatment. Chargari et al. reported that a series of older early stage BC patients (>70 years, cT1-2N0) treated with RT alone achieved a promising 7-year LC rate of 95.8% (10). In the 1980s, De Lena et al. reported a similar treatment response in a randomized trial of chemotherapy followed by surgery or RT for locally advanced BC (11). The treatment response of definitive CCRT is acceptable; however, no randomized phase III trials have compared the effectiveness of primary CCRT to standard treatment.
Modern radiation techniques, which include stereotactic irradiation or intensity-modulated RT (IMRT), may deliver tumoricidal doses without severe complications. A 3-year overall survival (OS) rate of 93% and LC rate of 92% with favorable cosmetics outcome was achieved with definitive whole-breast irradiation followed by stereotactic body RT boost (12). In this case series, we report five cases of BC from our institution, who refused surgical treatment and achieved good LC with primary CCRT.
Case presentation
From January 2010 to January 2014, five BC patients received definitive CCRT. All patients had the treatment strategy carefully explained to them, and provided their written informed consent to be included in this study. Clinical and image follow-up examinations were done regularly. This retrospective review was approved by our hospital Institutional Review Board, which found that it conformed to the provisions of the Declaration of Helsinki as revised in Edinburgh 2000. The stages presented were according to American Joint Committee on Cancer 7th edition (AJCC 7). The toxicities were graded according to the Common Terminology Criteria for Adverse Events, version 4.0.
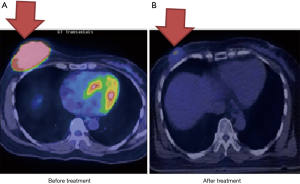
Case 1 was a 56-year-old woman who presented with a growing, non-tender and fixed left inner upper quadrant breast mass of more than 6 months’ duration in February 2013. Tumor biopsy reported invasive ductal carcinoma, cT2N1M0, AJCC 7 stage IIB with negative estrogen receptor (ER−), negative progesterone receptor (PR−), and positive human epidermal growth factor receptor 2 (HER2+). Positron emission tomography/computed tomography (PET/CT) scan showed an irregular left breast mass of 4.2 cm × 3.8 cm × 3 cm and involvement of the left axillary lymph node (Figure 1A). The clinical image staging was cT2N1M0, AJCC 7 stage IIB. The patient refused surgery and asked for nonsurgical treatment in our department. From May 2, 2013 to July 8, 2013, the patient received primary CCRT. Radiation consisted of 52.8 gray (Gy) in 24 fractions to the left breast and axilla lymph nodes with breast tumor bed boost to 76 Gy by tomotherapy and concurrent Tykerb (lapatinib). Adjuvant Herceptin (trastuzumab) and Xeloda (capecitabine) were given for another 4 months. Only Grade 1 skin reaction was noted during the whole treatment course. The tumor was not seen on the images during follow-up scans at 4 months after treatment (Figure 1B). The representative radiation field is shown in Figure 1C. The patient remains free of disease.
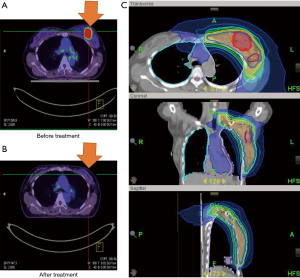
Case 2 was a 63-year-old woman whose left BC was diagnosed in October 2011. The tumor was located at 3 o’clock and 4 cm from the left nipple (3/4 cm). Excisional biopsy reported invasive ductal carcinoma, ER+, PR− and HER2+. The patient discontinued conventional treatment for one year and returned in December 2012 with a protruded tumor mass and palpable lymph nodes. PET/CT scan showed a large left breast mass (5.3 cm) with central necrosis, skin invasion and involvement of multiple axillary lymph nodes, cT4dN3bM0, AJCC 7 stage IIIC (Figure 2A). The patient continued to insist on non-surgical treatment. From January 4, 2013 to April 19, 2013, the patient received definitive CCRT to the left breast and regional lymph nodes by tomotherapy with concurrent Herceptin (trastuzumab), Taxotere (docetaxel) and epirubicin. RT was withheld after 55 Gy over 22 fractions due to Grade 2 neutropenia and dermatitis. After the patient recovered, a second RT cycle of 12 Gy in 6 fractions was completed in April 2013. The total radiation dose to the breast tumor bed was 67 Gy in 31 fractions. The follow-up PET scan showed good treatment response (Figure 2B). The patient continued with Xeloda (capecitabine) and Navelbine (vinorelbine) for one more year. Until now, she remains disease free with good cosmetic outcomes.
Case 3 was a 46-year-old woman whose right BC was diagnosed in 2010. Biopsy reported invasive ductal carcinoma with ER+, PR+ and HER2−. Initially, the patient presented with skin involvement and multiple palpable axillary lymph nodes (cT4aN2a, AJCC 7 stage IIIB) (Figure 3A). The patient refused surgical treatment, and she received neoadjuvant chemotherapy with Taxol (paclitaxel) followed by definitive RT of 68 Gy over 36 fractions delivered by IMRT from July 2010 to October 2010. The patient remained disease free for 3 years. In April 2013, local recurrence was noted and was successfully salvaged by re-irradiation of the recurrent tumor bed of 62 Gy in 31 fractions in July 2013 (Figure 3B).
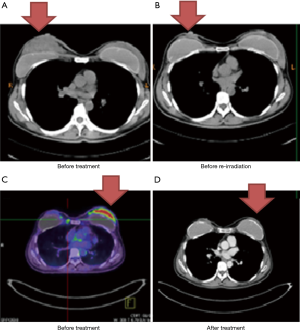
In 2014, the patient had a palpable left breast mass once again diagnosed with cancer. PET/CT scan showed a hypermetabolic left breast uptake of more than 5 cm (cT3N0M0, AJCC 7 stage IIB) (Figure 3C). From July 14, 2014 to October 13, 2014, the patient received a third RT course consisting of 64 Gy in 30 fractions by IMRT with concurrent biweekly cisplatin and maintenance Xeloda (capecitabine) for 1 more year after CCRT. The follow-up images showed no disease progression (Figure 3D).
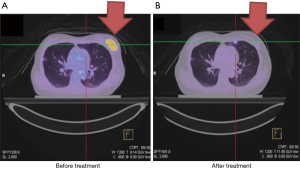
Case 4 was a 39-year-old woman who presented with two palpable masses at 12/1 cm and 2/1 cm from the left nipple. A biopsy in December 2010 indicated invasive ductal carcinoma with ER+, PR+ and HER2-. PET/CT showed a 2.5 cm tumor with axillary lymph node metastases, cT2N1, AJCC 7 stage IIB (Figure 4A). The patient refused surgery and asked for organ preservation treatment. From March 14, 2011 to May 16, 2011, the patient completed definitive RT of 48 Gy in 24 fractions to the left breast with a boost up to 68 Gy to the tumor bed by tomotherapy. Chemotherapy consisted of triweekly Taxotere (docetaxel) (100 mg) and epirubicin (90 mg). The patient had complete remission of the tumor (Figure 4B). She continued on tamoxifen for another 5 years and remained in good clinical and cosmetic condition.
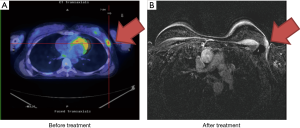
Case 5 was a 46-year-old woman with a palpable right breast mass noted in 2010. She did not receive any further examinations until the tumor size grew larger 2 years later. Biopsy of the tumor reported invasive ductal carcinoma, ER+, PR+ and HER2−. The patient refused surgical treatment and sought organ preservation treatment. PET/CT scan showed a 6 cm right breast tumor without lymph node involvement, cT3N0, AJCC 7 stage IIB (Figure 5A). The patient received definitive CCRT of 68 Gy in 36 fractions to the tumor bed by Tomotherapy concurrent with Taxotere (docetaxel) and epirubicin from May 15, 2012 to July 12, 2012. Treatment showed good therapeutic response (Figure 5B). Although the image examination was negative, the patient felt that a palpable nodule (less than 1 cm) persisted after irradiation. Eventually, a right mastectomy was performed in June 2013. Pathological studies showed no evident malignancies. The disease was well controlled and the patient remained in good health.
The characteristics of the five patients after CCRT are shown in Table 1. The five patients had an average age of 50 (range, 35–65) years. The BC stages were IIB to IIIC according to AJCC 7. All patients refused to undergo any type of surgical treatment and all received RT over 60 Gy. One patient had Grade 2 skin reactions and neutropenia, which led to splitting the treatment courses. The concurrent chemotherapy regimens included different combinations of hormone antagonists and/or cytotoxic chemotherapeutics. Although one patient eventually underwent mastectomy, the specimen showed no evidence of residual tumor. The treatment toxicities were mainly dermatological. All patients had good cosmetic outcome and remained in good health.
Table 1
| Case | Age | Diagnosis | Histology type | Location | TNM (AJCC 7) | Stage | ER, PR, Her2 | CCRT treatments | Response | Follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RT | Concurrent chemotherapy | ||||||||||
| 1 | 56 | 2013/3 | Invasive ductal carcinoma | Left | cT2N1M0 | IIB | ER −; |
2013/5–2013/7, 76 Gy/35 fx | 2013/5–2013/7: lapatinib. |
Complete response | Good cosmetic outcome. |
| 2 | 63 | 2011/10 | Invasive ductal carcinoma | Left | cT4dN3bM0 | IIIC | ER +; |
2013/1–2013/3 (hold RT after 55 Gy/22 fx due to Gr.2 skin reactions + neutropenia); 2013/4, 12 Gy/6 fx | 2013/1–2013/3: trastuzumab/docetaxel/epirubicin; |
Complete response | Disease free for more than 5 years with good cosmetic outcome |
| 3 | 46 | 2010/5 | Invasive ductal carcinoma | Right in 2010; |
cT4aN2a | IIIB | ER +; |
1st RT course to right breast, 2010/7–2010/10: 68 Gy/36 fx. |
2010/6–2010/10: paclitaxel; |
Local recurrence but successfully salvaged by re-RT | Disease free until now |
| 4 | 39 | 2010/12 | Invasive ductal carcinoma | Left | cT2N1M0 | IIB | ER +; |
2011/3–2011/5 to left breast cancer (68 Gy/34 fx) | 2011/3–2011/5: docetaxel/epirubicin | Complete response | Good cosmetics outcome |
| 5 | 46 | 2012/5 | Invasive ductal carcinoma | Right | cT3N0M0 | IIB | ER +; |
2012/5–2012/7 to right breast cancer (68 Gy/65 fx) | 2012/5–2012/7: docetaxel/epirubicin | Patient underwent right mastectomy on 2013/6: pathological complete response | Good cosmetics outcome, disease free until now |
−, negative; +, positive; Her2++, HER2 protein borderline; Her2+++, HER2 protein overexpression. CCRT, concurrent chemoradiotherapy; TNM, classification of malignant tumors by AJCC 7 definition; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; EB, electron beam; RT, radiotherapy.
Discussion
In our case series, we showed that primary CCRT can achieve good disease control. Our report was limited by the small case number (5 patients in the 4-year period), but all had a good LC rate. The fast treatment responses correlated with good treatment response. In a similar study, in patients who achieved clinical complete response (cCR) by neoadjuvant chemotherapy, both surgery and RT yielded similar 5-year OS rates (74% by RT vs. 76% by surgery, P=0.9) (13).
CCRT is one of the most effective treatment options for locally advanced disease. BC, although sensitive to chemotherapy and RT, is not routinely treated with CCRT. Few studies have reported the results of primary CCRT to treat BC in either the early stage or locally advanced stages (Table 2). In unresectable locally advanced or inflammatory BC, primary CCRT can achieve a significant cCR rate. Patients who achieve complete or partial response can reserve salvage operation for residual disease or disease recurrence without compromising survival (14,15). Karasawa et al. reported a 2-year LC rate of 73.6% and 65.9% OS rate for unresectable BC patients treated with primary CCRT (16). The all five cases we reported achieved cCR after definitive CCRT.
Table 2
| Studies | Study type | Patient number | Disease status | Treatment modality | RT dose | Chemotherapy/hormone therapy | Response rate |
|---|---|---|---|---|---|---|---|
| Mukai |
Single arm phase II | 108 | Stage I–IIIA | Neoadjuvant CCRT follow by surgery 12–16 weeks later | 45 Gy in 25 fractions (fx) with 10 Gy boost in 5 fx | Doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 (AC) ×4 follow by weekly paclitaxel 80 mg/m2 ×12 | pCR rate is 36%. |
| Chargari |
Retrospective | 396 (>70 years old) | T1-2 | Definitive hypo-fractionated radiotherapy | 32.5 Gy/5 fx/5 weeks follow by 13 Gy/2 fx boost | Hormone therapy for positive | Cause-specific survival: 96.4%; |
| De Lena |
Randomized | 132 | Locally advanced disease | Chemotherapy + RT |
– | Adriamycin plus vincristine (AV) for 10 cycles | Local control 75% in both groups |
| Shibamoto |
Retrospective | 18 | Operable IA to IIIC patients | Whole breast RT follow by Stereotactic body radiotherapy boost | 50 Gy in 25 fractions follow by boost with 18 to 25.5 Gy in 3 fx and 20 Gy in 8 fx | Hormone given o 9 patients. Chemotherapy given to 4 patients | 3-year overall survival: 93%; |
| Ring |
Retrospective | 136 | Operable breast carcinoma | Neoadjuvant chemotherapy follow by surgery or radiotherapy | 46 to 50 Gy follow by 11.1 to 17.5 Gy boost | Anthracycline-based regimens | Similar response between surgery and RT. |
| Bates |
Retrospective | 123 | (I) Non-metastatic locally advanced; |
Primary chemotherapy followed by radiotherapy and surgery for residual disease | 40 Gy in 15 fx with 10 Gy boost | AC ×6 or 5 FU 600 mg/m2, Epirubicin 75 mg/m2 & cyclophosphamide 600 mg/m2 every 3 weeks ×6 | Complete clinical response in 65%. |
| Kao |
Phase I/II trial | 33 | (I) Unresectable locally advanced or inflammatory breast cancers (T4N0-3M0-1); |
CCRT follow by mastectomy if operable | 60–70 Gy in a week-on/week-off schedule | Concurrent paclitaxel +/− vinorelbine | 47% had pCR. 4-year locoregional control: 83%. |
| Karasawa |
Retrospective | 39 | Unresectable T4 BC | CCRT | RT dose of 59–66 Gy | All received chemotherapy and/or endocrine therapy following CCRT | 2-year overall local control rate: 73.6%. |
CCRT, concurrent chemoradiotherapy; BC, breast cancer; RT, radiotherapy.
In our case series, patients did not receive surgery rather they were nervous or delayed after the diagnosis of BC. In another study, more than 20% of locally advanced BC patients delayed seeking medical intervention for more than 4 weeks (17). In a series from Denmark, 157 patients with locally advanced BC who delayed treatment had a high correlation of severe medical or psychiatric co-morbidity. More than 20% ignored their obvious symptoms of BC (18). Data from the Taiwan Cancer Registry database indicated that, in 35,095 patients, the risk factors for delaying BC treatment were being older than 75 years, lower income and high comorbidity index (19). As presented, for patients who are reluctant to agree to surgery, RT or CCRT is a satisfactory alternative.
Approximately one-third of BC patients suffer from local relapse. Re-irradiation is a controversial treatment, because of the high cumulative doses to the chest wall. Unfortunately, the 5-year LC rate for re-excision was only 33% (20). Wahl et al. conducted a multi-institutional study of re-irradiation of an average 48 Gy. Of the 81 patients enrolled, the cCR rate was 60%. Only 3 had late Grade 3 and just 1 experienced Grade 4 skin toxicity (21). A German retrospective study of 42 BC patients reviewed the role of repeat surgery and adjuvant re-irradiation of 60 Gy after a previous dose of 54 Gy. The 5-year LC and OS rates were 62% and 60%, respectively. Eight patients suffered from Grade 3 skin toxicities without any Grade 4 events (22). Those studies point out that chest wall re-irradiation decreased the local failure rate with acceptable toxicities. Similarly, in case 3, the patient had excellent disease control with good cosmetic outcomes after re-irradiation of 62 Gy.
Radiation for BC may induce skeletal, pleural or pulmonary changes. It is not uncommon for small residual masses to persist for months or even years after RT. Palpable or visible small post-radiation nodules on the CT of patients with treated head and neck cancers is a common sequelae of endothelial proliferation and fibrotic changes (23). A Japanese study of 50 lung cancer patients with persistent nodules after stereotactic body RT showed a LC rate of 84% after a median follow up of 52 months. The persistence of the nodules did not always correlate with an increased risk of recurrence (24). Non-increased PET uptake or stable sequential CT or magnetic resonance imaging findings are likely benign in patients whose clinical condition is stable. RT-induced thoracic changes are also seen physically or radiographically in skin, skeletal structures, the pleura (pleural thickening) and lungs (radiation pneumonitis and fibrosis) (25). In our case series, no patients had skin toxicities higher than Grade 2.
In conclusion, we have demonstrated that primary CCRT is an effective alternative treatment in patients who refuse surgery. In each case we reported, the disease was well-controlled with good quality of life and satisfactory cosmetic outcome. Until now, more than ten patients at our department had received this treatment strategy. All achieved good local control. We hope a randomized phase II or III trial to be launched to evaluate the real treatment efficacy.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu FC, Lin HT, Kuo CF, et al. Epidemiology and survival outcome of breast cancer in a nationwide study. Oncotarget 2017;8:16939-50. [PubMed]
- Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998;16:2672-85. [Crossref] [PubMed]
- Zucali R, Uslenghi C, Kenda R, et al. Natural history and survival of inoperable breast cancer treated with radiotherapy and radiotherapy followed by radical mastectomy. Cancer 1976;37:1422-31. [Crossref] [PubMed]
- He Y, Guo T, Guan H, et al. Concurrent chemoradiotherapy versus radiotherapy alone for locoregionally advanced nasopharyngeal carcinoma in the era of intensity-modulated radiotherapy: a meta-analysis. Cancer Manag Res 2018;10:1419-28. [Crossref] [PubMed]
- Datta NR, Stutz E, Liu M, et al. Concurrent chemoradiotherapy vs. radiotherapy alone in locally advanced cervix cancer: A systematic review and meta-analysis. Gynecol Oncol 2017;145:374-85. [Crossref] [PubMed]
- Glynne-Jones R, Nilsson PJ, Aschele C, et al. Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol 2014;40:1165-76. [Crossref] [PubMed]
- . N. I. Concurrent chemo-radiotherapy in the treatment of early breast cancer: Current status. Clin Cancer Investig J 2013;1-8.
- Lu Y, Huang H, Yang H, et al. Randomized controlled trial of late-course concurrent versus sequential chemoradiotherapy after mastectomy and axillary surgery in locally advanced breast cancer. Medicine (Baltimore) 2017;96:e8252 [Crossref] [PubMed]
- Mukai H, Watanabe T, Mitsumori M, et al. Final results of a safety and efficacy trial of preoperative sequential chemoradiation therapy for the nonsurgical treatment of early breast cancer: Japan Clinical Oncology Group Study JCOG0306. Oncology 2013;85:336-41. [Crossref] [PubMed]
- Chargari C, Kirova YM, Laki F, et al. The impact of the loco-regional treatment in elderly breast cancer patients: hypo-fractionated exclusive radiotherapy, single institution long-term results. Breast 2010;19:413-6. [Crossref] [PubMed]
- De Lena M, Varini M, Zucali R, et al. Multimodal treatment for locally advanced breast cancer. Result of chemotherapy-radiotherapy versus chemotherapy-surgery. Cancer Clin Trials 1981;4:229-36. [PubMed]
- Shibamoto Y, Murai T, Suzuki K, et al. Definitive Radiotherapy With SBRT or IMRT Boost for Breast Cancer: Excellent Local Control and Cosmetic Outcome. Technol Cancer Res Treat 2018;17:1533033818799355 [Crossref] [PubMed]
- Ring A, Webb A, Ashley S, et al. Is surgery necessary after complete clinical remission following neoadjuvant chemotherapy for early breast cancer? J Clin Oncol 2003;21:4540-5. [Crossref] [PubMed]
- Bates T, Williams NJ, Bendall S, et al. Primary chemo-radiotherapy in the treatment of locally advanced and inflammatory breast cancer. Breast 2012;21:330-5. [Crossref] [PubMed]
- Kao J, Conzen SD, Jaskowiak NT, et al. Concomitant radiation therapy and paclitaxel for unresectable locally advanced breast cancer: results from two consecutive phase I/II trials. Int J Radiat Oncol Biol Phys 2005;61:1045-53. [Crossref] [PubMed]
- Karasawa K, Saito M, Hirowatari H, et al. The role of chemoradiotherapy in patients with unresectable T4 breast tumors. Breast Cancer 2013;20:254-61. [Crossref] [PubMed]
- Nosarti C, Crayford T, Roberts JV, et al. Delay in presentation of symptomatic referrals to a breast clinic: patient and system factors. Br J Cancer 2000;82:742-8. [Crossref] [PubMed]
- El-Charnoubi WA, Svendsen JB, Tange UB, et al. Women with inoperable or locally advanced breast cancer -- what characterizes them? A retrospective review of 157 cases. Acta Oncol 2012;51:1081-5. [Crossref] [PubMed]
- Chen SJ, Kung PT, Huang KH, et al. Characteristics of the Delayed or Refusal Therapy in Breast Cancer Patients: A Longitudinal Population-Based Study in Taiwan. PLoS One 2015;10:e0131305 [Crossref] [PubMed]
- Siglin J, Champ CE, Vakhnenko Y, et al. Radiation therapy for locally recurrent breast cancer. Int J Breast Cancer 2012;2012:571946 [Crossref] [PubMed]
- Wahl AO, Rademaker A, Kiel KD, et al. Multi-institutional review of repeat irradiation of chest wall and breast for recurrent breast cancer. Int J Radiat Oncol Biol Phys 2008;70:477-84. [Crossref] [PubMed]
- Muller AC, Eckert F, Heinrich V, et al. Re-surgery and chest wall re-irradiation for recurrent breast cancer: a second curative approach. BMC Cancer 2011;11:197. [Crossref] [PubMed]
- Hermans R. Post-treatment imaging of head and neck cancer. Cancer Imaging 2004;4 Spec No A:S6-S15.
- Takenaka R, Shibamoto Y, Miyakawa A, et al. The Fate of Residual Tumor Masses That Persist After Stereotactic Body Radiotherapy for Solitary Lung Nodules: Will They Recur? Clin Lung Cancer 2016;17:406-11. [Crossref] [PubMed]
- Jung JI, Kim HH, Park SH, et al. Thoracic manifestations of breast cancer and its therapy. Radiographics 2004;24:1269-85. [Crossref] [PubMed]
Cite this article as: Kao P, Chi MS, Chi KH, Ko HL. Primary chemo-radiotherapy for breast cancer patients who refused surgical treatment: a case series. Ther Radiol Oncol 2019;3:24.


