
Stereotactic ablative radiotherapy for central and ultra-central lung tumors
Introduction
Non-small cell lung cancer (NSCLC) is the most common cause of cancer and cancer death worldwide, and stereotactic ablative radiotherapy (SABR) has emerged as a primary treatment modality for early-stage disease. SABR is considered standard-of-care for peripherally located, medically inoperable early-stage NSCLC (1-3). Regarding centrally located lung tumors, initial data published by Timmerman and colleagues in 2006 applying 3-fraction SABR showed high rates of severe toxicity, including some cases of fatal toxicity (4,5), although more recent data using 4–8 fraction SABR demonstrate safer outcomes (6-11). Still, these modern studies, including the prospective multi-institutional clinical trial RTOG 0813 (12), report some cases of severe toxicity and even some fatal toxicities (13), emphasizing that despite progress made over the past 13 years, it is important to exercise special caution when administering SABR to patients with central lung tumors.
Nonetheless, the volume of central lung tumor SABR is high enough at some academic medical centers that retrospective analyses have been conducted on tumors that have “ultra-central” location (7,14), defined as tumor abutting the trachea or proximal bronchial tree. In 2014, we published our experience treating 7 ultra-central lung tumors with SABR, 50 Gy in 4–5 fractions, and saw no significant toxicity on long-term follow-up (7). In contrast, a study by Haseltine and colleagues from New York published in 2016 showed a high rate of severe toxicity, including 4 cases (22%) of treatment-related fatal toxicity in their series of 18 patients with ultra-central lung tumors treated with SABR, 45–50 Gy in 5 fractions (14). An updated analysis demonstrated that anti-angiogenic therapy within 30 days of SABR significantly increased the risk of fatal hemorrhage in these patients (15). Tekatli and colleagues from VU Medical Center in Amsterdam also published on their experience treating 47 ultra-central NSCLC with hypofractionated radiotherapy of 60 Gy in 12 fractions. High rates of severe toxicity, including 10 (21%) likely treatment-related deaths, were observed (16). Based on these studies, hypofractionated radiotherapy for ultra-central lung tumors remains highly controversial. In this article, we will review SABR and hypofractionated radiotherapy for central and ultra-central lung tumors with a focus on factors associated with severe toxicity.
Anatomy of central and ultra-central lung tumors
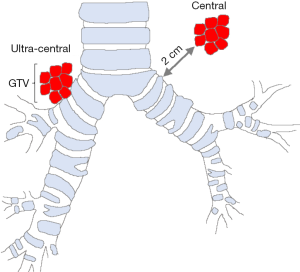
Lung tumor centrality was initially defined by Timmerman and colleagues in 2005 as a 2 cm zone around the proximal bronchial tree (Figure 1) (4). The proximal bronchial tree is defined as the region extending from the carina superiorly to the lower lobe bronchi inferiorly, which includes the upper, middle, lower and lingular bronchi and bronchus intermedius. The region encompassed by the proximal bronchial tree plus 2 cm margin has been referred to as a “no-fly zone” (17), referring to the exclusion of tumors in this location from subsequent clinical trials. The RTOG 0813 protocol defined centrality as Timmerman did (4), but it also included tumors with planned target volume (PTV) abutting the mediastinum (12). In 2014, our group defined ultra-central lung tumors as those with gross tumor volume (GTV) abutting the trachea or proximal bronchial tree (Figure 1), with generation 0 tumor defined as those abutting the trachea, generation 1 tumor as those abutting the carina or mainstem bronchi, and generation 2 tumors as those abutting the lobar or lingular bronchi or bronchus intermedius (7). Tekatli and colleagues proposed an alternative definition for ultra-central lung tumors in 2016, defining them as having PTV overlapping the trachea or mainstem bronchus (16). Other proposed definitions for ultra-centrality have included tumors with PTV abutting or overlapping the esophagus as allowed by the recently opened SUNSET trial (18).
SABR for central lung tumors
In 2006, Timmerman and colleagues published the results of their prospective phase II clinical trial at Indiana University treating early-stage medically inoperable NSCLC with SABR, 60 Gy in 3 fractions (~54 Gy in 3 fractions with dose heterogeneity correction) (4,5). Local tumor control at 2 years was impressive at 95%; however, 2-year overall survival was lower at 54.7%. Subset analysis revealed 22 (31%) from a total of 70 patients had centrally located tumors lying within 2 cm of the proximal bronchial tree. The grade 3 to 5 toxicity rate was 10.4% in patients with peripheral tumors vs. 27.3% in those with centrally located tumors. Among the 22 central lung tumor patients, six experienced grade 3 to 5 toxicity, of which four suffered fatal grade 5 toxicity, including pneumonia, respiratory failure and hemoptysis. This study was the first to highlight the potential danger of treating centrally located lung tumors with SABR, and given its results the subsequent RTOG 0236 study excluded patients with centrally located lung tumors altogether (1).
Since the Indiana University experience, however, multiple retrospective studies have been published suggesting that SABR for central lung tumors can achieve both high local tumor control and low rates of severe toxicity. In general, these studies employed greater fractionation and lower biologically effective dose (BED) than the 54 Gy in 3 fractions treatment regimen that was becoming a standard for peripheral tumors. In our report on the Stanford experience using 50 Gy in 4–5 fractions, among 34 patients treated for centrally located lung tumors we observed only one grade ≥3 toxicity, which was a case of grade 4 radiation pneumonitis (7). Local tumor control in our patients was 90% at 2 years.
Rowe et al. published the Yale experience treating 47 patients with centrally located lung tumors and also treated patients predominantly with 50 Gy in 4 fractions (6). Local tumor control at two years was 94%, and the rate of grade ≥3 toxicity was 10.6% which included one case of hemoptysis that contributed to respiratory failure and death.
Chang et al. published the MD Anderson experience treating 27 NSCLC patients with centrally/superiorly located lung cancer with 40–50 Gy in 4 fractions, with tumors located within 2 cm of the bronchial tree, great vessels, esophagus, heart, trachea, pericardium, brachial plexus or vertebral body (10). Local tumor control was 100% in patients treated with 50 Gy, but only 57% in those treated with 40 Gy. Regarding toxicity, three patients (11.1%) developed grade 2–3 dermatitis and chest wall pain, and one patient developed brachial plexus neuropathy and partial arm paralysis. There were no cases of grade ≥3 pneumonitis or grade ≥4 toxicity.
Haasbeek and colleagues reported the VU University experience treating 63 patients with central lung tumors with 60 Gy in 8 fractions, and reported a 3% rate of grade 3 dyspnea, 3% rate of grade 3 chest wall pain and no definitive grade 4 or 5 toxicity, although they noted that grade 5 toxicity could not be excluded with certainty in the 14% of patients who died of cardiopulmonary causes (8). Three-year local tumor control was excellent at 92.6%.
Bral et al. published the University Hospital Brussels experience treating 40 lung cancer patients with SABR, 17 of whom had centrally located NSCLC. Patients with central tumors were treated with 60 Gy in 4 fractions, which was generally well tolerated except for 3 cases of grade 3 pneumonitis and 1 fatality from hemorrhage that followed a stenting procedure for bronchial stenosis (11).
Ma and colleagues published the University of Buffalo experience treating 11 patients with centrally located lung tumors with 26–30 Gy in 1 fraction and 31 patients with 50–60 Gy in 5 fractions (19). Local tumor control at 1 year was 100% for the single fraction group and 96% for the multi-fraction group. They observed four treatment-related grade 3–4 toxicities (9.5%) and no grade 5 toxicities, with two grade 3 toxicities in the multi-fraction group, one grade 3 and one grade 4 toxicity in the single fraction group. The grade 4 toxicity was a bronchopulmonary hemorrhage in a patient treated with SABR to the right hilum.
Generally speaking, these retrospective series indicate that reducing the dose intensity of SABR appears to lead to a more tolerable toxicity profile than Timmerman and colleagues observed (4,5), while still maintaining very high local tumor control (20).
Regarding prospective data, a phase I clinical trial from Washington University treated patients with biopsy-proven NSCLC within 2 cm of the proximal bronchial tree using 5 fractions in a dose-escalated fashion starting with 9 Gy per fraction and ending at 12 Gy per fraction (9). Based on this analysis, 11 Gy per fraction was taken forward into a phase II prospective trial that demonstrated a 2-year local control rate of 85%; however, in 41 patients evaluable for late cardiac and pulmonary toxicity, 11 (27%) developed grade 3, five (12%) developed grade 4, and one (4%) died of a grade 5 toxicity (9). The case of fatal hemoptysis occurred in a patient with tumor involving the pulmonary artery.
Kimura and colleagues from the Japanese Radiation Oncology Study Group also performed a prospective phase I study in which they treated nine patients with central lung tumors with 60 Gy in 8 fractions, and saw no dose limiting toxicities (21). The trial planned to increase dose to 64 Gy in 8 fractions; however, this was ultimately not done due to the challenge of meeting normal tissue dose constraints.
Based in part on the above studies, the RTOG conducted a multi-institutional prospective phase I/II clinical trial to study the safety and efficacy of central lung tumor SABR more comprehensively (12). In RTOG 0813, medically inoperable early-stage NSCLC patients, with GTV within 2 cm of the proximal bronchial tree or with PTV abutting the mediastinum, were treated with SABR. A starting dose of 50 Gy in 5 fractions was planned, with dose escalation up to 60 Gy in 5 fractions, as tolerated (12). There was no grade 3 or higher toxicities reported in the eight patients treated on the 50 Gy dose arm; the 52.5 Gy arm (7 patients) and 55 Gy arm (14 patients) each included a grade 5 toxicity. The 57.5 Gy and 60 Gy cohorts were taken forward into the phase II component of the trial to further evaluate efficacy and toxicity (12). In the reported phase II results, 71 patients were treated; the grade ≥3 toxicity rate was 21% and the rate of grade 5 toxicity was 5.6%. These toxicities were typically late with all grade 5 toxicities (4 in total) occurring >1 year after treatment and included a possible esophageal ulcer that eroded into a major vessel, and three bronchopulmonary hemorrhages. There were only three cases (4.8%) of grade ≥3 adverse events within 1 year of SABR, all of which were grade 3 pulmonary toxicity. The 2-year local control rate in the phase II study was 87.9–89.4% and 2-year OS was 67.9–72.7%. These results suggest that a 5-fraction SABR regimen appears reasonably safe for most patients, although severe adverse events occurring >1 year after treatment were higher than would be expected for peripheral lung tumor treatment.
SABR for ultra-central lung tumors
Our group at Stanford defined ultra-central lung tumors as those abutting the trachea or proximal bronchial tree (7). In our retrospective study, we included 34 patients with central tumors treated with SABR, among which seven were treated for ultra-centrally located lung tumors (6 with NSCLC, 1 with oligometastatic renal cell carcinoma). We observed that patients treated with 50 Gy in 4–5 fractions with ultra-central tumors achieved superb long-term outcomes with high rates of local tumor control and no grade ≥2 toxicity.
Raman et al. published a retrospective study detailing Princess Margaret Hospital’s experience treating 26 ultra-central lung tumors with 60 Gy in 8 fractions for a majority of the patients, and saw a 7.9% (n=2) rate of grade 2 toxicity and no grade 3, 4 or 5 toxicities (22). Local tumor control was 100% at 2 years.
Sood and colleagues presented the Kansas retrospective experience of 62 patients with ultra-central lung tumors treated with 10-fraction stereotactic hypofractionated radiotherapy (23). The 1-year local tumor control rate was 84%, and the overall toxicity rate was low with one case of grade 3 esophagitis and no definite grade 4 or 5 toxicities.
Henke and colleagues treated 5 patients with ultra-central lung tumors on a prospective phase I clinical trial using MR-guided adaptive radiotherapy (SMART) to a dose of 50 Gy in five fractions. This resulted in highly conformal treatment plans and no grade 3 or higher acute toxicities, although one patient experienced an esophageal stricture 15 months after treatment (24).
Nguyen and colleagues from UC Davis published a retrospective study on their experience treating ultra-central lung tumors with SABR and compared outcomes to patients with other centrally located lung tumors treated with SABR (25). In total, 14 patients with ultra-central tumors and 54 patients with other central tumors were reviewed. Two-year local tumor control was 89% (25), similar to the phase II results from RTOG 0813 (12). The predominant dose fractionation was 50 Gy in 5 fractions (25), which achieved high local tumor control. However, in contrast to the Stanford study on patients treated with a similar dosing regimen (7), the UC Davis study found significantly higher rates of grade ≥2 toxicity in patients with ultra-central lung tumors compared to those with other central lung tumors (25). In total, 57.6% of ultra-central patients experienced grade ≥2 toxicity, with two patients (14%) developing grade ≥3 toxicity and one patient experiencing grade 5 respiratory failure. Maximum point doses to the central structures—airway, great vessels and esophagus—were significantly higher in patients with ultra-central lung tumors than in those with other central lung tumors. Patients in whom the RTOG 0813 central airway maximum point dose constraint was exceeded experienced significantly higher rates of grade ≥2 pulmonary toxicity.
Haseltine et al. also conducted a retrospective study of central and ultra-central lung tumors treated at the Memorial Sloan Kettering Cancer Center (MSKCC) with SABR (14). Patients were mostly treated with 45–50 Gy in 5 fractions. The authors defined centrality per RTOG 0813 (12), and ultra-centrality as Stanford did (7); 108 patients were included in the study, including 18 with ultra-central tumors abutting the proximal bronchial tree (PBT) (14). Patients were additionally categorized as those with GTV ≤1 cm from the PBT, and those with GTV >1 cm from the PBT. OS and LC were similar between the two groups, but patients with GTV ≤1 cm from the PBT had significantly higher rates of severe (grade ≥3) adverse events than those with GTV >1 cm from the PBT (31% vs. 7%).
Fatal toxicity occurred in 4 patients, all of whom harbored tumors abutting the PBT (14). Among them, two patients received long courses of bevacizumab, which were started well before radiotherapy, held during SABR, and then continued afterwards. Both of these patients were relatively young at ~50 years old, being treated for oligometastatic disease, and had SABR to small ultra-central lung tumors <3 cm in size (with GTV ≤20 cc). It has been reported that SABR or hypofractionated radiotherapy in the setting of anti-angiogenic treatment can lead to severe toxicity (15,26,27). The combinatorial use of these therapies may have thus contributed to these fatal adverse events.
The other two patients in the Haseltine study who experienced grade 5 toxicity died of pneumonia, with one dying of sepsis and the other of acute respiratory failure (14). In both of these cases, the underlying etiology was unclear, but there was clinical suspicion that radiotherapy played a role. In both of these cases, a left lower lobe T1–T2 primary NSCLC tumor was treated with 45 Gy in 5 fractions; bilateral lung V20 was ~8.5%. Based on this dosimetry, risk of radiation pneumonitis would have been expected to be low in these patients, although this risk can increase in the setting of interstitial lung disease (28,29).
Wang and colleagues updated the MSKCC experience treating ultra-central lung tumors with hypofractionated stereotactic radiotherapy (15). Eighty-eight patients with ultra-central lung tumors were retrospectively reviewed (15), 76 of whom had tumors abutting the proximal bronchial tree and 23 with PTV overlapping the esophagus (some patients met both criteria). Patients were treated with hypofractionated radiotherapy in 5, 8 or 15 fractions with a BED ≥ 84 Gy. At a median follow-up of 19.6 months, the grade ≥3 overall toxicity rate was 22%. Ten (11%) patients experienced grade 5 toxicity possibly related to SABR, including six who experienced fatal pulmonary hemorrhage, four of whom received anti-angiogenic treatment within 30 days of SABR. Indeed, the probability of fatal pulmonary hemorrhage was found to be significantly higher in patients who were treated with anti-angiogenic agents within a month of SABR compared to those who did not receive these agents (P<0.001). The authors thus recommended that the combination of SABR and anti-angiogenic therapy be avoided in patients with ultra-central lung tumors (15).
Another recent study, published by Tekatli and colleagues, retrospectively reviewed outcomes in 47 medically inoperable patients with single primary or recurrent ultra-central NSCLC tumors treated with a hypofractionated regimen of 60 Gy in 12 fractions (16). In this study, ultra-central tumors were defined as those with PTV overlapping the trachea or main stem bronchus. At a median follow-up of 29.3 months, 38% of patients experienced grade ≥3 toxicity with 21% of patients having a possible or likely grade 5 toxicity, with the most common fatal toxicity being due to pulmonary hemorrhage.
Patients in the Tekatli study were typically of advanced age and poor performance status (16). Tumors were typically large, with median PTV size being 104.5 cc. In 94% of cases, the PTV overlapped the main stem bronchus, while in 43% of cases it overlapped the trachea, and in 36% of cases there was overlap of the PTV with both the trachea and main stem bronchus. Overall, patients in this study were high-risk based on these tumor and patient-related factors. Indeed, these patients were offered this treatment regimen because they were deemed unfit for either surgery or conventional chemoradiotherapy (16).
Another potential risk factor retrospectively identified by Tekatli and colleagues was anti-coagulant or anti-platelet medication use (16). The authors noted that 71% of patients who experienced fatal hemorrhage after hypofractionated radiotherapy were taking oral anti-coagulant or anti-platelet drugs. This was higher than the ~50% of patients who were using these drugs in the overall treated cohort. It is possible that the increased bleeding risk associated with these medications exacerbated the toxicity effect of hypofractionated radiotherapy in these patients. However, this hypothesis requires further study.
Tekatli and colleagues also determined that fatal hemorrhage cases occurred in 3 of 4 (75%) cases of interstitial lung disease (16). Interstitial lung disease is thought to be a risk factor for high grade pulmonary complications after radiation therapy (28,29), but it is unclear how it relates to the pathogenesis of acute hemorrhage. Therefore, this hypothesis is another topic that merits further study.
Similar to the regimen employed by Tekatli et al. (16), UT Southwestern and Stanford published their prospective dose escalation study treating NSCLC patients with 50 to 60 Gy in 15 fractions where 19 patients were treated with 60 Gy in 15 fractions, and these treatments were generally well-tolerated (30). The Stanford group (Pollom et al.) also performed a retrospective study on patients treated between 52.5 Gy and 60 Gy in 15 fractions (31), 30 of whom were treated with 60 Gy. Both these studies were predominantly comprised of patients who were not candidates for surgery or concurrent chemoradiation (30,31), similar to the patient population included in Tekatli’s retrospective study (16).
The discrepancy in toxicity outcomes between Tekatli’s study (16) and the published 15-fraction data (30,31) is unclear, and perhaps may be attributed to dose to the proximal pulmonary artery. Pollom et al. frequently included paratracheal and subcarinal lymphadenopathy abutting the trachea and mainstem bronchus, but avoided cases in which the tumor encased the proximal pulmonary artery (31). Another possibility is that tumors were not treated as conformally in the Tekatli study, which used intensity modulated radiotherapy (IMRT) with 4-7 coplanar beams (16), compared to the Pollom’s study which typically used multi-beam or arc-based IMRT (31). Tekatli and colleagues also performed a retrospective dosimetric analysis on their cohort. While no significant differences were reported, there was an apparent trend towards higher maximum point doses (as high as 84 Gy) in the 7 patients who experienced fatal hemoptysis (16). Thus, differences in toxicity between the Tekatli et al. data using 60 Gy in 12 fractions (16) and the Pollom et al. data using predominantly 60 Gy in 15 fractions (31) could be in part due to different dosimetry, an issue that is exacerbated by a lack of consensus for tumor and normal tissue constraints for ultra-central lung tumor cases.
Another possible explanation for the high toxicity rate in Tekatli’s study is that 53% of patients had endobronchial tumor present (16). Notably, 43% of patients who developed fatal hemorrhage after hypofractionated radiotherapy were treated for an endobronchial lesion. The authors also noted that malignancy was not pathologically proven in 11 (23%) patients, among which five experienced significant bleeding or clinical deterioration during bronchoscopy that required termination of the procedure. Thus, it is possible that patients in Tekatli’s study were at especially high risk for acute pulmonary hemorrhage because patients typically underwent bronchoscopic biopsy shortly before radiotherapy of predominantly endobronchial tumors (16).
It is important to note that there is limited data available for patients with tumors abutting the esophagus treated with SABR. In the retrospective study by Wang et al., 2 of 23 (8.7%) patients with PTV overlapping the esophagus developed tracheoesophageal fistula (15). Sodji and colleagues also performed a retrospective study of 15 patients treated with SABR for lung tumors located within 2 cm of the esophagus, where the esophagus received at least 80% of the prescribed dose (32). Different dose fractionations were employed, with the most common being 40 Gy in 4 fractions. Seven (47%) patients developed grade 2 esophagitis at a median of 10 days after SABR, which was temporary and resolved, and no patients experienced grade ≥3 toxicity. Patients who experienced symptomatic esophagitis typically received treatment with BED >80 Gy (32). More data are required to clarify our understanding of esophageal dose tolerance in the setting of hypofractionated radiotherapy, and how to recommend treatment in cases where the tumor is near the esophagus. The recently launched SUNSET trial, a multi-center prospective phase I dose escalation study, includes patients with PTV abutting or overlapping the esophagus in its eligibility criteria (18), and will hopefully shed further light on this topic.
Regarding prospective data for ultra-central lung SABR, Lindberg and colleagues presented preliminary results of the Nordic HILUS-Trial (33), a phase II study of SABR to tumors located within 1 cm of the proximal bronchial tree. Patients were divided into two groups, one with 42 patients with tumors near the mainstem bronchus, and 31 with tumors close to the lobar bronchus. Twenty-one patients (28%) experienced grade 3–5 adverse effects, with seven patients likely experiencing fatal treatment-related toxicity, six of whom suffered from fatal hemoptysis after a median of 15.5 months post-treatment and one experiencing severe pneumonitis which contributed to death. Overall, grade 4–5 toxicities were much more common in patients with tumor close to a mainstem bronchus than in those with tumor near a lobar bronchus (19% vs. 3%) (33). The increased rate of toxicity associated with tumor near the mainstem bronchus is consistent with Tekatli et al.’s data (16).
Patients with tumors near mainstem bronchi may be at especially high risk for severe, life-threatening toxicity. To examine ultra-central lung SABR specifically, the SUNSET phase I dose escalation study was recently opened in Canada, which defines ultra-central tumors as those with PTV touching or overlapping the proximal bronchial tree, esophagus or pulmonary artery. The starting dose was set at 60 Gy in 8 fractions delivered daily (18). The results of this trial will help guide our treatment practices for ultra-central lung tumors.
Conclusions
SABR has emerged as the primary modality of treatment for patients with inoperable early-stage NSCLC. However, prospective data from Timmerman and colleagues established that the severe toxicity rate can be unacceptably high in patients with centrally located lung tumors who received a particularly dose intensive regimen (4,5). This led to the exclusion of these patients from RTOG 0236 (1). Multiple subsequent institutional studies, mostly retrospective, examined SABR dose fractionation for central lung tumors using predominantly 4–8 fraction regimens (6-11), while maintaining BED ≥100 Gy (34). These studies reported more favorable toxicity outcomes, while maintaining high local tumor control. This culminated in RTOG 0813, a multi-institutional phase I/II trial with data suggesting that a 5-fraction SABR regimen appears reasonably safe for most patients (12). Thus, central tumor SABR is now performed more commonly in specialized academic radiation oncology centers.
We demonstrated in 2015 that SABR could also be used to treat patients with ultra-central lung tumors, which we defined as those abutting the trachea or proximal bronchial tree (7). We observed favorable tumor control and no severe toxicities during long-term follow-up; however, our retrospective cohort included only seven patients with ultra-central tumors. While some other retrospective studies have observed similar findings (22,23), others have shown alarming rates of severe and sometimes fatal toxicity in patients with ultra-central lung tumors treated with SABR (14,15,25) or even with 12-fraction hypofractionated IMRT (16). The most common grade 5 toxicity in these studies has been fatal pulmonary hemorrhage. Multiple factors, including the use of VEGF inhibitors, anti-coagulant or anti-platelet medications, tumor invasion or encasement of proximal bronchi or pulmonary vasculature, and/or high doses to these structures, are probably contributing to this high toxicity rate. Dosimetric factors that may be important include high- and intermediate-dose conformity, dose gradients across bronchi and great vessels, and dose-volume parameters for these central structures. Reporting these details in publications of clinical experiences, whether prospective or retrospective, will help develop practice guidelines for treating tumors in these high-risk locations. The SUNSET trial recently began enrolling patients with ultra-central lung tumors in a prospective fashion and should provide further clarity and guidance (18). As we await results of this trial, we should continue to maintain special caution when considering patients with ultra-central lung tumors for SABR or hypofractionated stereotactic radiotherapy.
Acknowledgments
Funding: This work was funded with support from the National Cancer Institute under award number K08CA238711 (AA Chaudhuri), and the Cancer Research Foundation Young Investigator Award (AA Chaudhuri).
Footnote
Conflicts of Interest: AA Chaudhuri has received speaker honoraria and travel support from Varian Medical Systems, Roche Sequencing Solutions, and Foundation Medicine, receives research support from Roche Sequencing Solutions, and is a consultant/advisor for Geneoscopy, Roche, and Tempus Labs. M Diehn receives research funding from Varian Medical Systems, holds ownership interest in CiberMed, and is a consultant/advisory board member for Roche, AstraZeneca, and BioNTech. BW Loo Jr receives research support from Varian Medical Systems, and is a board member of TibaRay. Kevin Chen has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Nyman J, Hallqvist A, Lund JA, et al. SPACE - A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol 2016;121:1-8. [Crossref] [PubMed]
- Ball D, Mai GT, Vinod S, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol 2019;20:494-503. [Crossref] [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009;75:677-82. [Crossref] [PubMed]
- Rowe BP, Boffa DJ, Wilson LD, et al. Stereotactic body radiotherapy for central lung tumors. J Thorac Oncol 2012;7:1394-9. [Crossref] [PubMed]
- Chaudhuri AA, Tang C, Binkley MS, et al. Stereotactic ablative radiotherapy (SABR) for treatment of central and ultra-central lung tumors. Lung Cancer 2015;89:50-6. [Crossref] [PubMed]
- Haasbeek CJ, Lagerwaard FJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol 2011;6:2036-43. [Crossref] [PubMed]
- Roach MC, Robinson CG, DeWees TA, et al. Stereotactic Body Radiation Therapy for Central Early-Stage NSCLC: Results of a Prospective Phase I/II Trial. J Thorac Oncol 2018;13:1727-32. [Crossref] [PubMed]
- Chang JY, Balter PA, Dong L, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;72:967-71. [Crossref] [PubMed]
- Bral S, Gevaert T, Linthout N, et al. Prospective, risk-adapted strategy of stereotactic body radiotherapy for early-stage non-small-cell lung cancer: results of a Phase II trial. Int J Radiat Oncol Biol Phys 2011;80:1343-9. [Crossref] [PubMed]
- Bezjak A, Paulus R, Gaspar LE, et al. Safety and Efficacy of a Five-Fraction Stereotactic Body Radiotherapy Schedule for Centrally Located Non-Small-Cell Lung Cancer: NRG Oncology/RTOG 0813 Trial. J Clin Oncol 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Corradetti MN, Haas AR, Rengan R. Central-airway necrosis after stereotactic body-radiation therapy. N Engl J Med 2012;366:2327-9. [Crossref] [PubMed]
- Haseltine JM, Rimner A, Gelblum DY, et al. Fatal complications after stereotactic body radiation therapy for central lung tumors abutting the proximal bronchial tree. Pract Radiat Oncol 2016;6:e27-33. [Crossref] [PubMed]
- Wang C, Rimner A, Gelblum DY, et al. Analysis of Toxic Effects With Antiangiogenic Agents Plus Stereotactic Body Radiation in Ultracentral Lung Tumors. JAMA Oncol 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Tekatli H, Haasbeek N, Dahele M, et al. Outcomes of Hypofractionated High-Dose Radiotherapy in Poor-Risk Patients with "Ultracentral" Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:1081-9. [Crossref] [PubMed]
- Chang JY, Li QQ, Xu QY, et al. Stereotactic ablative radiation therapy for centrally located early stage or isolated parenchymal recurrences of non-small cell lung cancer: how to fly in a "no fly zone". Int J Radiat Oncol Biol Phys 2014;88:1120-8. [Crossref] [PubMed]
- Giuliani M, Mathew AS, Bahig H, et al. SUNSET: Stereotactic Radiation for Ultracentral Non-Small-Cell Lung Cancer-A Safety and Efficacy Trial. Clin Lung Cancer 2018;19:e529-32. [Crossref] [PubMed]
- Ma SJ, Syed YA, Rivers CI, et al. Comparison of single- and five-fraction schedules of stereotactic body radiation therapy for central lung tumours: a single institution experience. J Radiother Pract 2017;16:148-54. [Crossref] [PubMed]
- Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol 2017;7:295-301. [Crossref] [PubMed]
- Kimura T, Nagata Y, Harada H, et al. Phase I study of stereotactic body radiation therapy for centrally located stage IA non-small cell lung cancer (JROSG10-1). Int J Clin Oncol 2017;22:849-56. [Crossref] [PubMed]
- Raman S, Yau V, Pineda S, et al. Ultracentral Tumors Treated With Stereotactic Body Radiotherapy: Single-Institution Experience. Clin Lung Cancer 2018;19:e803-10. [Crossref] [PubMed]
- Sood SS, Shen X, Chen AM, et al. Ultracentral Thoracic Reirradiation Using 10 Fraction Stereotactic Body Radiation Therapy for Recurrent Non-small Cell Lung Cancer Tumors: Preliminary Toxicity and Efficacy Outcomes. Int J Radiat Oncol Biol Phys 2017;99:E497
- Henke LE, Olsen JR, Contreras JA, et al. Stereotactic MR-guided online adaptive radiation therapy (SMART) for ultracentral thorax malignancies: Results of a phase 1 trial. Adv Radiat Oncol 2018;4:201-9. [Crossref] [PubMed]
- Nguyen KNB, Hause DJ, Novak J, et al. Tumor Control and Toxicity Following SBRT for Ultracentral, Central and Paramediastinal Lung Tumors. Pract Radiat Oncol 2019;9:e196-202. [Crossref] [PubMed]
- Chaudhuri AA, Chen JJ, Carter JN, et al. Tracheal Diverticulum Following Paratracheal Hypofractionated Radiotherapy in the Setting of Prior and Subsequent Bevacizumab. Cureus 2016;8:e578 [PubMed]
- Pollom EL, Deng L, Pai RK, et al. Gastrointestinal Toxicities With Combined Antiangiogenic and Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys 2015;92:568-76. [Crossref] [PubMed]
- Chen H, Senan S, Nossent EJ, et al. Treatment-Related Toxicity in Patients With Early-Stage Non-Small Cell Lung Cancer and Coexisting Interstitial Lung Disease: A Systematic Review. Int J Radiat Oncol Biol Phys 2017;98:622-31. [Crossref] [PubMed]
- Chaudhuri AA, Binkley MS, Rigdon J, et al. Pre-treatment non-target lung FDG-PET uptake predicts symptomatic radiation pneumonitis following Stereotactic Ablative Radiotherapy (SABR). Radiother Oncol 2016;119:454-60. [Crossref] [PubMed]
- Westover KD, Loo BW Jr, Gerber DE, et al. Precision hypofractionated radiation therapy in poor performing patients with non-small cell lung cancer: phase 1 dose escalation trial. Int J Radiat Oncol Biol Phys 2015;93:72-81. [Crossref] [PubMed]
- Pollom EL, Qian Y, Durkee BY, et al. Hypofractionated intensity-modulated radiotherapy for patients with non-small-cell lung cancer. Clin Lung Cancer 2016;17:588-94. [Crossref] [PubMed]
- Sodji Q, Ko R, Binkley MS, et al. Esophagitis in Patients Treated With Thoracic Stereotactic Ablative Radiation Therapy (SABR) to Tumors within 2 Cm of the Esophagus. Int J Radiat Oncol Biol Phys 2018;102:e666 [Crossref]
- Lindberg K, Nyman J, Riesenfeld Kallskog V, et al. Long-term results of a prospective phase II trial of medically inoperable stage I NSCLC treated with SBRT - the Nordic experience. Acta Oncol 2015;54:1096-104. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. [Crossref] [PubMed]
Cite this article as: Chaudhuri AA, Chen K, Diehn M, Loo BW Jr. Stereotactic ablative radiotherapy for central and ultra-central lung tumors. Ther Radiol Oncol 2019;3:18.


