
A proton primer to stereotactic lung radiotherapy
Introduction
Stereotactic body radiation therapy (SBRT), or stereotactic ablative radiotherapy as it is otherwise known, is defined as “an external beam radiation therapy method used to very precisely deliver a high dose of radiation to an extracranial target within the body, using either a single dose or a small number of fractions” (1). Although surgical resection is still widely viewed as the gold standard for curative treatment in early stage non-small cell lung cancer (NSCLC), SBRT is being utilized more commonly, especially in patients who refuse surgery or are deemed to be non-operative candidates (2). Phase I data in this setting showing acceptable safety and toxicity profiles were first published in 2003 by Timmerman et al. (3). Since then, convincing data showing excellent local control rates of 85–97.6% using this technique have been reported (4-7), which have also led to favorable direct comparisons of SBRT to surgical resection (6,7).
Conventionally, SBRT is delivered in early stage NSCLC using photon beams. Although this method has proven to be safe and effective in many cases, there are still technical and toxicity-related concerns, particularly when treating tumors located near critical organs (8). Central tumors are commonly defined as being within 2 cm of the proximal bronchial airways or immediately adjacent to nearby critical organs including the heart, esophagus, and spinal cord (9,10). Serious SBRT toxicities such as pericardial effusion, pneumonitis, pneumonia, hemoptysis, bronchial stenosis, fistula and death have been reported when treating central tumors (10-13). Other critical organs at risk (OARs) can include the brachial plexus and chest wall. The treatment of large tumors, multiple tumors simultaneously and re-irradiation are additional clinical scenarios associated with increased risk of toxicity when utilizing SBRT.
The use of proton radiotherapy has become increasingly common as a means to potentially reduce toxicity associated with SBRT. Historically, stereotactic body proton therapy (SBPT) was delivered using passive scattering techniques, but in recent years more modern and conformal active scanning techniques are being utilized. Protons possess a dosimetric advantage compared to photons in which the target dose is delivered to a specific depth, or “Bragg Peak”, followed by a rapid dose fall off which results in little or no exit dose. This allows for sparing of OARs located close to lung cancer target volumes (14) and therefore has the potential to reduce acute and chronic toxicities when compared to conventional photon SBRT. Proton therapy also allows for a significant reduction in integral dose to patients which can further reduce late toxicities.
Purpose
The purpose of this review is to explore the currently available literature relating to stereotactic radiotherapy in early stage NSCLC in order to compare the use of SBPT to SBRT and to highlight future directions for research.
Dosimetry and feasibility studies
Several studies have demonstrated a potential dosimetric advantage favoring SBPT over SBRT (15-22). SBPT plans significantly decreased mean lung doses and V5Gray to the ipsilateral and contralateral lungs (16-18,20-22). Many of these studies have also demonstrated lower doses delivered to critical OARs such as the heart and esophagus (17-20,22). Although some of the dosimetric SBPT advantages seen were modest, it must be noted that the vast majority of patients in these studies were treated using passive scattering techniques. The dosimetric advantages of SBPT over SBRT could have been underestimated by the use of passive or double scattering techniques rather than pencil beam scanning, which allows for the most conformal dose distribution with protons (23).
Westover et al. reported their experience of 15 patients with 20 stage 1 NSCLCs who were generally high risk for treatment, most of whom had interstitial lung disease, multiple primary tumors, or prior thoracic RT. These patients were treated with SBPT to a median dose of 45 cobalt gray equivalent (CGE) in 14 fractions. They reported 3 cases of rib fracture and 1 case of grade 3 pneumonitis. They also reported 2-year overall survival and local control rates of 64% and 100%, respectively (24). Nakayama et al. reported their experience of 55 patients treated with double scattering, most commonly to 66 CGE in 10 fractions with 2-year overall survival and local control outcomes reported at 97.8% and 97%, respectively. Only 2 patients had deterioration of lung function and another 2 patients developed grade 3 pneumonitis (25).
The oldest and largest clinical experience was reported by Bush et al. at Loma Linda (26). This 12-year experience included 111 patients treated with 1 of 3 dose levels: 51, 60 or 70 CGE in 10 fractions. The authors reported increased 4-year overall survival rates with each progressive dose level (18%, 32% and 51% respectively). Peripheral T1 tumors showed local control rates of 96% at 4 years. This clinical experience also reported excellent safety outcomes with none of the patients suffering from significant treatment-related pneumonitis or decreased pulmonary function. There were, however, 4 patients who developed a rib fracture, all of whom had tumors in close proximity to the chest wall (26).
A large meta-analysis was also recently conducted that compared particle beam stereotactic radiotherapy to SBRT using photons, however, this analysis included studies in which patients were treated with carbon ion therapy, which has somewhat different dosimetric properties than protons. Chi et al. compared 72 SBRT studies to 9 hypofractionated particle therapy studies and reported 3-year local control rates favoring particle based therapy on multivariate analysis and a decreased rate of severe (grade 3+) toxicity with particle therapy (0.9% vs. 3.4%, P=0.001). While overall and progression free survival were statistically better in the particle studies on univariate analysis, these differences did not remain significant on multivariate analysis. This analysis was limited given that most studies included were single arm, single institutional, observational studies with heterogeneous patient populations and treatment planning and delivery techniques, which could have introduced a significant selection bias (27).
Randomized evidence
One randomized trial attempted to compare SBRT to SBPT in early stage NSCLC, however, it closed early due to poor accrual (28). Reasons for premature closure included lack of 3D volumetric imaging in the SBPT arm, lack of insurance coverage in the SBPT arm, strict inclusion of only “high risk patients” in the trial and patient treatment preferences (28,29). For the 21 patients successfully enrolled, median survival time was not reached in the SBPT group and was 28 months in the SBRT group. The 3-year local control was similar in both groups (90% vs. 87.5%). Three-year overall survival in the SBPT group was 90% vs. 27.8% in the SBRT group, but this difference was not statistically significant given the small patient numbers in the trial. Only one patient in the study experienced a grade 3 toxicity (skin fibrosis in the SBPT arm) with no grade 4 or 5 toxicities being reported in either arm. The authors concluded that if a similar study were to be attempted in the future, it would require improvements in volumetric imaging for SBPT and improved cooperation with insurance companies (28).
Uncertainties and cost
Due to the physical characteristics of protons, there is a significant amount of dose delivery uncertainty just distal to the Bragg Peak. Therefore, clinicians attempt to orient beams to avoid end-ranging into critical OARs. This end range uncertainty can arise due to a variety of factors which include inaccuracy in the CT Hounsfield unit conversion to proton stopping power, treatment set up uncertainties and inter-treatment changes in patient anatomy (30-32).
The effect of these uncertainties becomes magnified with the high doses per fraction and reduced number of fractions delivered with stereotactic lung radiotherapy. Particularly relevant to lung cancer, tumor motion due to normal physiologic respiration adds another degree of uncertainty. Motion management strategies for proton therapy include the use of 3-dimensional volumetric on-board imaging to ensure beam delivery accuracy and utilization of breath hold techniques which have been shown to be feasible (33,34). Uncertainty of tumor motion is further compounded by the fact that the use of active scanning proton delivery can introduce a second dimension of complexity known as the “interplay effect.” This is further defined when “relative motion between a tumor and a scanning proton beam results in degradation of the dose distribution” (35,36). Strategies to account for this include utilization of Monte Carlo and robust optimization algorithms (36,37). As the interplay effect is understood to be a random error, another successful strategy is the use of “dose repainting.” This is defined as splitting the delivered dose into 2 or more fractions delivered sequentially during a single treatment (as opposed to conventional twice daily fractions that are typically delivered at least 6 hours apart). This process effectively increases the number of fractions, which is more likely to smear out the interplay effect by negating the random error, ultimately reducing treatment planning uncertainty (38).
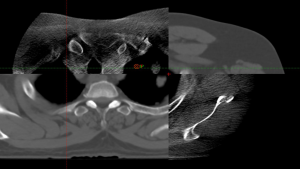
Robust optimization can also be used to account for inter-treatment changes in patient anatomy, which can have significant dosimetric and clinical consequences. Proton radiotherapy is especially sensitive to these changes compared to photon radiotherapy (39). A clinical example of this is shown in Figure 1. Another unique issue when treating lung tumors is the drastic change in density from lung to tumor tissue. This density gradient has a greater effect on proton particles than photons. It can also be accounted for by utilizing Monte Carlo simulations which account for sudden changes in tissue density (30,31). Finally, the lack of volumetric on-board imaging has been cited as an obstacle for the use of SBPT in lung cancer (28), however, as proton treatment gantries with cone beam CAT scan capabilities become more widely available this will likely be less relevant going forward (33).
The financial cost of delivering proton treatment has been a major hurdle in treating patients using protons, especially in privately funded health care systems. Theoretically, if proton treatment is able to decrease dose to OARs, this could potentially lead to decreased costs associated with the clinical management of radiation-induced toxicities. However, proton therapy is currently significantly more expensive compared to conventional photon therapy at most centers, and its’ use in individual cases must be clinically justified. Peeters et al. conducted an in depth cost effectiveness analysis comparing conventional photon treatments to proton treatments and found that the cost of running a proton facility was 2.6 times higher than that of running a photon facility, and that the cost per fraction of proton treatment was 3.2 times higher than photon therapy. Interestingly, and particularly relevant to this discussion, the authors found that the cost difference between proton and photon therapy was smallest when treating stage 1 NSCLC and they observed a linear increase in treatment cost as the number of fractions increased. This could suggest that the use of fewer fractions, as is delivered in stereotactic radiotherapy, would offer a potential cost benefit with the use of protons (40). Additionally, the potential for acute and late toxicity reduction with SBPT may prove it to be more cost effective in the long term.
Treatment recommendations
The use of SBPT in the management of early stage NSCLC should be considered on an individual patient basis and should account for tumor and non-tumor related factors such as medical comorbidities. The Thoracic Subcommittee of the International Particle Therapy Co-operative Group (PTCOG) published treatment guidelines in 2016, which outline specific tumor characteristics which could warrant the use of SBPT (41). They recommend that small, peripheral tumors not be treated with SBPT, as the potential benefit compared to SBRT is marginal at best. They do, however, recommend consideration of SBPT for larger tumors due to dosimetric advantages and possible reductions in chest wall and rib toxicity. There is also a recommendation for consideration of SBPT in patients with multiple tumors based on a case report by Shi et al. (42).
The PTCOG recommendation also suggests consideration for proton therapy for central tumors which abut critical OARs such as the esophagus, heart, major vessels, spinal cord or airways in order to reduce potential toxicity. They also recommend that tumors close to the brachial plexus be considered for SBPT in order to reduce treatment-related neuropathies (41).
Outside of the PTCOG recommendations, other treatment scenarios in which SBPT may be considered include cases in which dose reductions to the chest wall and ribs are desired. Welsh et al. demonstrated SBPT’s ability to achieve superior dosimetry and similar PTV target while reducing chest wall dose. They concluded that the dosimetric reduction seen with SBPT versus SBRT could result in fewer adverse clinical outcomes such as chest wall pain and rib fracture (43). SBPT can also be considered in patients with poor lung function, as measured by low pulmonary function testing, as there can be significant reductions in mean lung dose and low dose lung volumes with SBPT compared to SBRT (16-18,20-22). Proton therapy may also be helpful in patients with oligo-metastatic or oligo-progressive disease who are actively receiving or have recently received systemic therapy in an attempt to reduce the dose to the lungs and other critical OARs, thus potentially reducing toxicities that may be additive or synergistic when combining radiotherapy and systemic therapy. Finally, SBPT can be considered in re-irradiation scenarios where sparing dose to OARs is critical.
Conclusions and future directions
Currently, consideration for the use of SBPT in early stage NSCLC should be limited to certain complex treatment scenarios, including central tumors close to OARs, large or multiple tumors and re-irradiation cases. Additional randomized trials comparing SBRT directly to SBPT are needed to fill current knowledge gaps but are unlikely in the immediate future. Future trials should evaluate the effectiveness of SBPT with pencil beam scanning delivered with on-board volumetric imaging (23). Further work is also needed to better understand the inherent uncertainties associated with proton therapy. Finally, more cost-effectiveness research is required to determine whether the use of protons can significantly reduce the costs of treatment-related toxicities and establish proton therapy as a cost effective treatment modality. This, in turn, could result in increased cooperation and willingness to cover treatment costs by insurance companies. SBPT in early stage NSCLC shows great promise in certain clinical scenarios, but its ultimate value as a treatment modality in radiation oncology is yet to be established.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: MV Mishra and CB Simon 2nd receive a speaker’s honorarium from Varian Medical Systems. CBS serve as an unpaid editorial board members of Therapeutic Radiology and Oncology from Aug 2018 to Aug 2020. MVM serve as an unpaid editorial board members of Therapeutic Radiology and Oncology from Sep 2019 to Aug 2021.The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Potters L, Kavanagh B, Galvin JM, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) Practice Guideline for the Performance of Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys 2010;76:326-32. [Crossref] [PubMed]
- Haasbeek CJA, Palma D, Visser O, et al. Early-stage lung cancer in elderly patients: A population-based study of changes in treatment patterns and survival in the Netherlands. Ann Oncol 2012;23:2743-7. [Crossref] [PubMed]
- Timmerman R, Papiez L, McGarry R, et al. Extracranial Stereotactic Radioablation: Results of a Phase I Study in Medically Inoperable Stage I Non-small Cell Lung Cancer. Chest 2003;124:1946-55. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic Body Radiation Therapy for Inoperable Early Stage Lung Cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Roach MC, Robinson CG, DeWees TA, et al. Stereotactic body radiation therapy (SBRT) for central early stage non-small cell lung cancer: results of a prospective phase I/II trial. J Thorac Oncol 2018;13:1727-32. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage 1 non-small-cell lung cancer: a pooled analysis of two randomized trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Timmerman RD, Paulus R, Pass HI, et al. Stereotactic Body Radiation Therapy for Operable Early-Stage Lung Cancer: Findings from the NRG Oncology RTOG 0618 Trial. JAMA Oncol 2018;4:1263-6. [Crossref] [PubMed]
- Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol 2017;7:295-301. [Crossref] [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive Toxicity When Treating Central Tumors in a Phase II Study of Stereotactic Body Radiation Therapy for Medically Inoperable Early-Stage Lung Cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Chang JY, Li QQ, Xu QY, et al. Stereotactic Ablative Radiation Therapy for Centrally Located Early Stage or Isolated Parenchymal Recurrences of Non-Small Cell Lung Cancer: How to Fly in a “No Fly Zone”. Int J Radiat Oncol Biol Phys 2014;88:1120-8. [Crossref] [PubMed]
- Song SY, Choi W, Shin SS, et al. Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer 2009;66:89-93. [Crossref] [PubMed]
- Milano MT, Chen Y, Katz AW, et al. Central thoracic lesions treated with hypofractionated stereotactic body radiotherapy. Radiother Oncol 2009;91:301-06. [Crossref] [PubMed]
- Corradetti MN, Haas AR, Rengan R. Central-Airway Necrosis after Stereotactic Body-Radiation Therapy. N Engl J Med 2012;366:2327-9. [Crossref] [PubMed]
- Simone CB 2nd, Rengan R. The use of proton therapy in the treatment of lung cancers. Cancer J 2014;20:427-32. [Crossref] [PubMed]
- Mou B, Beltran CJ, Park SS, et al. Feasibility of Proton Transmission-Beam Stereotactic Ablative Radiotherapy versus Photon Stereotactic Ablative Radiotherapy for Lung Tumors: A Dosimetric and Feasibility Study. PLoS One 2014;9:e98621 [Crossref] [PubMed]
- Macdonald OK, Kruse JJ, Miller JM, et al. Proton Beam Radiotherapy versus Three-Dimensional Conformal Stereotactic Body Radiotherapy in Primary Peripheral, Early-Stage Non-Small-Cell Lung Carcinoma: A Comparative Dosimetric Analysis. Int J Radiat Oncol Biol Phys 2009;75:950-8. [Crossref] [PubMed]
- Hoppe BS, Huh S, Flampouri S, et al. Double-scattered proton-based stereotactic body radiotherapy for stage I lung cancer: A dosimetric comparison with photon-based stereotactic body radiotherapy. Radiother Oncol 2010;97:425-30. [Crossref] [PubMed]
- Seco J, Panahandeh HR, Westover K, et al. Treatment of Non-Small Cell Lung Cancer Patients with Proton Beam-Based Stereotactic Body Radiotherapy: Dosimetric Comparison with Photon Plans Highlights Importance of Range Uncertainty. Int J Radiat Oncol Biol Phys 2012;83:354-61. [Crossref] [PubMed]
- Georg D, Hillbrand M, Stock M, et al. Can protons improve SBRT for lung lesions? Dosimetric considerations. Radiother Oncol 2008;88:368-75. [Crossref] [PubMed]
- Register SP, Zhang X, Mohan R, et al. Proton Stereotactic Body Radiation Therapy for Clinically Challenging Cases of Centrally and Superiorly Located Stage I Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2011;80:1015-22. [Crossref] [PubMed]
- Kadoya N, Obata YO, Kato T, et al. Dose-Volume Comparison of Proton Radiotherapy and Stereotactic Body Radiotherapy for Non-Small-Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2011;79:1225-31. [Crossref] [PubMed]
- Wink KC, Roelofs E, Simone CB 2nd, et al. Photons, protons or carbon ions for stage I non-small cell lung cancer - results of the multicentric ROCOCO in silico study. Radiother Oncol 2018;128:139-46. [Crossref] [PubMed]
- Chang JY, Zhang X, Knopf AConsensus Guidelines for Implementing Pencil-Beam Scanning Proton Therapy for Thoracic Malignancies on Behalf of the PTCOG Thoracic and Lymphoma Subcommittee, et al. Int J Radiat Oncol Biol Phys 2017;99:41-50. [Crossref] [PubMed]
- Westover KD, Seco J, Adams JA. Proton SBRT for Medically Inoperable Stage 1 NSCLC. J Thorac Oncol 2012;7:1021-5. [Crossref] [PubMed]
- Nakayama H, Sugahara S, Tokita M. Proton Beam Therapy for Patients with Medically Inoperable Stage I Non-Small-Cell Lung Cancer at the University of Tsukuba. Int J Radiat Oncol Biol Phys 2010;78:467-71. [Crossref] [PubMed]
- Bush DA, Cheek G, Zaheer S, et al. High-Dose Hypofractionated Proton Beam Radiation Therapy Is Safe and Effective for Central and Peripheral Early-Stage Non-Small Cell Lung Cancer: Results of a 12-Year Experience at Loma Linda University Medical Center. Int J Radiat Oncol Biol Phys 2013;86:964-8. [Crossref] [PubMed]
- Chi A, Chen H, Wen S, et al. Comparison of particle beam therapy and stereotactic body radiotherapy for early stage non-small cell lung cancer: A systematic review and hypothesis-generating meta-analysis. Radiother Oncol 2017;123:346-54. [Crossref] [PubMed]
- Nantavithya C, Gomez DR, Wei X, et al. Phase 2 Study of Stereotactic Body Radiation Therapy and Stereotactic Body Proton Therapy for High-Risk, Medically Inoperable, Early-Stage Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2018;101:558-63. [Crossref] [PubMed]
- Gomez DR, Li H, Chang JY. Proton therapy for early-stage non-small cell lung cancer (NSCLC). Transl Lung Cancer Res 2018;7:199-204. [Crossref] [PubMed]
- Paganetti H. Range uncertainties in proton therapy and the role of Monte Carlo simulations. Phys Med Biol 2012;57:R99-117. [Crossref] [PubMed]
- Knopf AC, Lomax A. In vivo proton range verification: a review. Phys Med Biol 2013;58:R131-60. [Crossref] [PubMed]
- Liebl J, Paganetti H, Zhu M, et al. The influence of patient positioning uncertainties in proton radiotherapy on proton range and dose distribution. Med Phys 2014;41:091711 [Crossref] [PubMed]
- Veiga C, Janssens G, Teng CL, et al. First clinical investigation of cone beam computed tomography and deformable registration for adaptive proton therapy for lung cancer. Int J Radiat Oncol Biol Phys 2016;95:549-59. [Crossref] [PubMed]
- Dueck J, Knopf AC, Lomax A, et al. Robustness of the Voluntary Breath-Hold Approach for the Treatment of Peripheral Lung Tumors Using Hypofractionated Pencil Beam Scanning Proton Therapy. Int J Radiat Oncol Biol Phys 2016;95:534-41. [Crossref] [PubMed]
- Kang M, Huang S, Solberg TD, et al. A study of the beam-specific interplay effect in proton pencil beam scanning delivery in lung cancer. Acta Oncol 2017;56:531-40. [Crossref] [PubMed]
- Dowdell S, Grassberger C, Sharp GC, et al. Interplay effects in proton scanning for lung: A 4D Monte Carlo study assessing the impact of tumor and beam delivery parameters. Phys Med Biol 2013;58:4137-56. [Crossref] [PubMed]
- Engwall E, Fredriksson A, Glimelius L. 4D robust optimization including uncertainties in time structures can reduce the interplay effect in proton pencil beam scanning therapy. Med Phys 2018;45:4020-9. [Crossref] [PubMed]
- Poulsen PR, Eley J, Langner U, et al. Efficient Interplay Effect Mitigation for Proton Pencil Beam Scanning by Spot-Adapted Layered Repainting Evenly Spread out Over the Full Breathing Cycle. Int J Radiat Oncol Biol Phys 2018;100:226-34. [Crossref] [PubMed]
- Li H, Zhang X, Park P, et al. Robust optimization in intensity-modulated proton therapy to account for anatomy changes in lung cancer patients. Radiother Oncol 2015;114:367-72. [Crossref] [PubMed]
- Peeters A, Grutters JPC, Pijls-Johannesma M, et al. How costly is particle therapy? Cost analysis of external beam radiotherapy with carbon-ions, protons and photons. Radiother Oncol 2010;95:45-53. [Crossref] [PubMed]
- Chang JY, Jabbour SK, De Ruysscher D, et al. Consensus Statement on Proton Therapy in Early-Stage and Locally Advanced Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2016;95:505-16. [Crossref] [PubMed]
- Shi W, Nichols RC Jr, Flampouri S, et al. Proton-based chemoradiation for synchronous bilateral non-small-cell lung cancers: A case report. Thoracic Cancer 2013;4:198-202. [Crossref] [PubMed]
- Welsh J, Amini A, Ciura K, et al. Evaluating Proton Stereotactic Body Radiotherapy to Reduce Chest Wall Dose in the Treatment of Lung Cancer. Med Dosim 2013;38:442-7. [Crossref] [PubMed]
Cite this article as: Sharma AM, Simone CB 2nd, Remick J, Kowalski E, Mishra MV. A proton primer to stereotactic lung radiotherapy. Ther Radiol Oncol 2019;3:16.


