Solitary plasmacytoma of bone over sacrum: a case report
Introduction
Plasma cell malignancies are a group of cancers which proliferated from plasma cells. These cancers can be classified into single lesion or multiple lesions. The former is solitary plasmacytoma while the latter is multiple myeloma. Solitary plasmacytomas can occur in bone or soft tissue, and it has a high risk of progression to multiple myeloma. Here, we reviewed a patient who had solitary plasmacytoma of bone (SPB) over sacrum undergone radiotherapy.
Case presentation
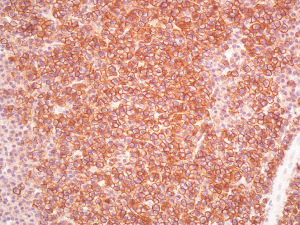
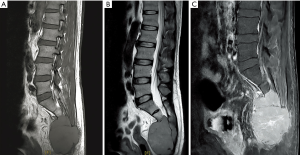
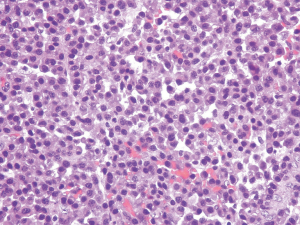
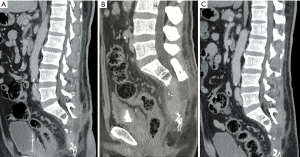
A 46-year-old man visited our hospital due to lower back pain for 3 months. His past medical history included only hypertension without any trauma. The pain, which radiated to his lower limbs, was accompanied by numbness. The aggravating factors of the pain were prolonged standing and sitting. He could not bear ambulation for long periods due to intermittent claudication. Body weight loss of about 12 kg in 3 months was also noted. There was no urine or fecal incontinence. L-spine and pelvic magnetic resonance imaging (MRI) scan was arranged and revealed a large tumor (8.8 cm × 6.2 cm) over the sacrum. The occupying lesion over the pelvis involved the sacrum (S2 to S4), with hypointensity on both T1-weighted and T2-weighted images. It was homogeneously contrast enhanced (Figure 1A,B,C). He then received surgical intervention with partial tumor removal on May 31, 2016. Pathology report showed plasmacytoma from sacrum bone and soft tissue. Diffuse plasmacytoid cells with eccentric nuclei were found in the soft tissue with bony invasion. Immunohistochemical study demonstrated cytokeratins (CK) (−), Cluster of Differentiation (CD) 138 (+), Kappa light chain (−), Lambda light chain (+), and CD 20 (−) for tumor cells (Figures 2-4). β2 microglobulin, after tumor excision, was 3270 nanograms per milliliter.
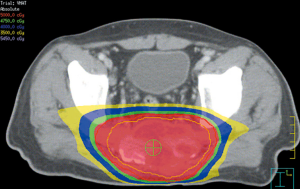
Under the diagnosis of SPB, partial tumor removal and adjuvant radiotherapy were arranged. Computed tomography (CT)-simulation was performed on June 13, 2016. A series of 5-mm-slice contrast-enhanced CT images was acquired with a CT simulator. The CT images were transferred to the planning system for target delineation. To account for set-up error, planning target volume (PTV) included a 5-millimeter margin around the clinical target volume (CTV). Six megavoltage (MV) photon with volumetric modulated arc therapy (VMAT) technique was used. The prescribed dose was 50 grays (Gy) in 2 Gy daily fractions to PTV. Treatment was administered 5 days per week, for a total of 25 fractions. VMAT was optimized to cover 100% of the PTV with 100% of the prescribed dose. Due to dose constraint of rectum, rectal dose was adjusted to 45 Gy, which was 12% less than 50 Gy. Radiotherapy was started on post-operation day 20 (from June 20, 2016 to July 22, 2016) (Figures 5,6). After treatment, the patient experienced no acute reactions. Neither dermatitis nor gastrointestinal discomfort was noted.
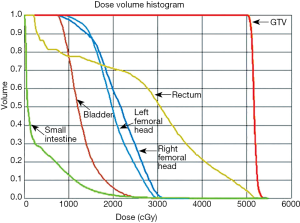
An oncologist was consulted, and bone marrow biopsy was suggested to exclude multiple myeloma. The bone marrow biopsy showed hypocellularity (20–30%) of bone marrow and scattered plasma cells (<5%) infiltrating the interstitium. Multiple myeloma was ruled out. After consultation, no adjuvant chemotherapy was suggested.
After completion of radiotherapy, the patient was followed up for 24 months until July 2018. Pelvic CT was performed every 3 months, which showed contrast-enhancing soft tissue density lesion in sacrum, with decreasing solid and cystic components (Figure 7A,B,C). No newly-developed lesion was noted. β2 microglobulin level was in the normal range during the follow-up period. No distant metastasis or newly diagnosed multiple myeloma was noted. His reported quality of life was satisfactory, and his lower back pain improved with no intermittent claudication.
Discussion
SPB is also called osseous plasmacytoma. It is one type of solitary plasmacytoma. SPB mostly occurs in axial skeleton, vertebral bodies and the skull (1,2). SPBs occur more frequently in males, and the male-to-female ratio is about 2:1. The median age of patients with SPB is around 55 years, which is about 10 years lower than that of patients with multiple myeloma (3). The incidence of SPB is approximately 3.5 per 1 million (4). A review of the literature provides incidences of SPB for different ethnic groups. However, data on the Asian population is lacking. Only on study conducted in Taiwan shows that the incidence of SPB is 6.2% among all plasma neoplasms (1). M protein, which is a monoclonal paraprotein, was found to be a poor prognostic factor if it existed for more than one year and was associated with development of multiple myeloma within 2.2 years with or without treatment (5).
The diagnosis of SPB requires biopsy-proven solitary tumor of bone with evidence of clonal plasma cells in single area of bone and without additional lesions of spine on MRI. The pathologic immunohistochemical study may be CD 138 and lambda light chain positive. There should be no evidence of systemic myeloma, such as anemia, hypercalcemia or renal function impairment (6). Moreover, there should be no monoclonal plasma cells in bone marrow aspirate, serum or urine (3).
Imaging reports have indicated that SPB is mostly located in the vertebrae and long bones (7). Before treatment, SPB lesions often show homogeneous low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. MRI is more advantageous than CT in terms of enhancement of the mass. The marked homogeneous enhancement on MRI may be helpful for the diagnosis of pelvic SPB (8). In our case, the pelvic MRI lesion was hypointensed on both T1-weighted and T2-weighted images, and was homogeneously contrast enhanced. The pelvic mass showed high signal intensity in the short tau inversion recovery phase.
SPB has a high risk of progression to multiple myeloma. The primary treatment is localized radiation therapy to avoid this progression, which occurs in 50% of cases within 5 years, 65–84% in 10 years, and 65–100% in 15 years (4,6). The optimal therapy for SPB should aim to achieve long-term local control with minimal morbidity of multiple myeloma. Definitive local radiotherapy could provide fabulous local control, may translate into a persistent remission and even cure. In our case, a long term follow up 5 to 10 years is still needed.
Surgery is not the first choice for the treatment of SPB, except if there is structural instability of the bone, retropulsed bone, or rapidly progressive symptoms from cord compression (9). Tsutsumi et al. presented four patients with solitary spinal extradural plasmacytoma who received tumor resection, and the results showed neurological symptom improvement (10). Total en bloc spondylectomy also resulted in clinical improvements, but an experienced team was required. Kadokura et al. presented a patient with left back pain. Radiological evaluation revealed a chest wall tumor (11). CT-guided biopsy was performed and pathology report showed SPB. Preoperative radiotherapy at a dose of 40 Gy was administered to the tumor. Afterward, he underwent complete resection of the tumor. Adjuvant chemotherapy with melphalan and prednisolone was given. During a 2-year follow-up, there was no tumor recurrence. Kumar et al. reported a patient presenting with headache on the right side for three months (12). Brain MRI demonstrated an osseous lesion (32 mm × 16 mm) along the posterior cranial fossa, involving the right petrous temporal bone. Treatment methods included right retromastoid suboccipital craniotomy and subtotal excision of the tumor. Histopathology revealed plasmacytoma and positive CD 138. The final diagnosis was SPB of right temporal bone. Adjuvant radiotherapy to the tumor was given at a dose of 50 Gy in 25 fractions. During a 2-year follow-up, there was no evidence of tumor recurrence.
Radiotherapy has been shown to provide excellent local control of SPBs. The largest retrospective study (n=258) included patients with SPB (n=206) or extramedullary plasmacytoma (n=52) (10). Treatments included radiotherapy alone (n=214), radiotherapy plus chemotherapy (n=34), and surgery alone (n=8). Five-year overall survival was 74%, disease free survival was 50%, and local control was 85%. Patients receiving localized radiotherapy had a lower rate of local recurrence (12%) than those not receiving radiotherapy.
Many retrospective studies have included only small numbers of patients, due to the low prevalence of SPB (6) (Table 1). The standard treatment is definitive local radiotherapy. In the treatment field, using MRI to define GTV is recommended. The strengths of MRI are high soft tissue contrast and multiplanar imaging. CT scan should also be conducted to detect bone abnormality. Thus, the treatment field should be determined by comparing both CT image and MRI. However, the treatment field for planning remains controversial. Some authors have suggested including whole bone in the radiation field to avoid marginal recurrence, while others have recommended that only part of the bone be included. For CTV, we encompassed at least 1.5–2 cm margin on MRI, with 5–10 mm margin for PTV. Some authors have suggested a dose of 40–50 Gy for smaller lesions (<5 cm) and >50 Gy for larger lesions (>5 cm) (2,3). Suh et al. suggested doses of <35 Gy for lesions <5 cm and 40–50 Gy for larger lesions (>5 cm) (16). In a multicenter Rare Cancer Network study, 258 patients with bone (n=206) and extramedullary (n=52) SPB were treated at the Princess Margaret Hospital. They found no dose-response relationship for doses >30 Gy, even for larger tumors (10). In our case, we chose a dose of 50 Gy in 25 fractions due to the large tumor size (8.8 cm × 6.2 cm).
Table 1
| Patient numbers | Median follow-up time | Overall survival rate (%) | Progression free survival | |
|---|---|---|---|---|
| Nahi |
124 | – | 56 (8 years) | 65% (2 years) |
| Ozsahin |
206 | 56 months | 52 | 28% |
| Tsang |
32 | 8 years | 65 | 44% |
| Kilciksiz |
57 | 2.4 years | 73 | 3.5 years |
| Reed |
59 | 5.3 years | 76 | 56% |
Use of adjuvant chemotherapy is controversial. In some studies, adjuvant chemotherapy has revealed no benefit in terms of disease control or prevention of progression to multiple myeloma (1,6). In one prospective study, a benefit of combined treatment using local radiotherapy with doses ranging from 40–50 Gy (28 patients) followed by melphalan and prednisone every 6 weeks for 3 years was demonstrated compared to radiotherapy alone (25 patients) (17). After a median follow-up of 8.9 years, disease free survival and overall survival improved in patients treated with combined therapy. Moreover, 22 patients remained alive and free of disease in the combined treatment group compared to only 13 patients in the radiotherapy alone group (P<0.01). Another retrospective study showed that melphalan-based chemotherapy can prevent multiple myeloma development. Patients who are not responsive to radiotherapy can be treated with chemotherapy, with a similar approach to that used in the treatment of multiple myeloma (18). However, some studies have reported no benefit for adjuvant chemotherapy. Moreover, a Greek myeloma study group showed that the addition of chemotherapy increases toxicity without offering any survival advantage over radiotherapy (11).
In conclusion, radiotherapy is the main treatment for SPB. Surgery is recommended when there is structural instability of the bone or rapidly progressive symptoms from cord compression. There is no sufficient evidence to suggest use of adjuvant chemotherapy for SPB (3). Further studies are needed to determine the effects of surgery and chemotherapy.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2019.02.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shih LY, Dunn P, Leung WM, et al. Localised plasmacytomas in Taiwan: comparison between extramedullary plasmacytoma and solitary plasmacytoma of bone. Br J Cancer 1995;71:128-33. [Crossref] [PubMed]
- Nahi H, Genell A, Walinder G, et al. Incidence, characteristics, and outcome of solitary plasmacytoma and plasma cell leukemia. Population-based data from the Swedish Myeloma Register. Eur J Haematol 2017;99:216-22. [Crossref] [PubMed]
- Soutar R, Lucraft H, Jackson G, et al. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary. Br J Haematol 2004;124:717-26. [Crossref] [PubMed]
- Jawad MU, Scully SP. Skeletal Plasmacytoma: Progression of disease and impact of local treatment; an analysis of SEER database. J Hematol Oncol 2009;2:41. [Crossref] [PubMed]
- Wilder RB, Ha CS, Cox JD, et al. Persistence of myeloma protein for more than one year after radiotherapy is an adverse prognostic factor in solitary plasmacytoma of bone. Cancer 2002;94:1532-7. [Crossref] [PubMed]
- Kilciksiz S, Karakoyun-Celik O, Agaoglu FY, et al. A Review for Solitary Plasmacytoma of Bone and Extramedullary Plasmacytoma. ScientificWorldJournal 2012;2012:895765 [Crossref] [PubMed]
- Tikku G, Jain M, Mridha A, et al. Solitary Bone Plasmacytoma Progressing into Retroperitoneal Plasma Cell Myeloma with No Related End Organ or Tissue Impairment: A Case Report and Review of the Literature. Turk J Haematol 2014;31:286-9. [Crossref] [PubMed]
- Wang Y, Zhu XL, Peeroo MW, et al. Pelvic Solitary Plasmacytoma: Computed Tomography and Magnetic Resonance Imaging Findings with Histopathologic Correlation. Korean J Radiol 2015;16:146-53. [Crossref] [PubMed]
- Katodritou E, Terpos E, Symeonidis AS, et al. Clinical features, outcome, and prognostic factors for survival and evolution to multiple myeloma of solitary plasmacytomas: A report of the Greek myeloma study group in 97 patients. Am J Hematol 2014;89:803-8. [Crossref] [PubMed]
- Ozsahin M, Tsang RW, Poortmans P, et al. Outcomes and patterns of failure in solitary plasmacytoma: a multicenter Rare Cancer Network study of 258 patients. Int J Radiat Oncol Biol Phys 2006;64:210-7. [Crossref] [PubMed]
- Kadokura M, Tanio N, Nonaka M, et al. A Surgical Case of Solitary Plasmacytoma of Rib Origin with Biclonal Gammopathy. Jpn J Clin Oncol 2000;30:191-5. [Crossref] [PubMed]
- Kumar R, Kumar N, Mohindro S, et al. Solitary plasmacytoma of temporal bone: A rare case report. Asian J Neurosurg 2017;12:95-7. [Crossref] [PubMed]
- Tsang RW, Gospodarowicz MK, Pintilie M, et al. Solitary plasmacytoma treated with radiotherapy: impact of tumor size on outcome. Int J Radiat Oncol Biol Phys 2001;50:113-20. [Crossref] [PubMed]
- Kilciksiz S, Celik OK, Pak Y, et al. Clinical and prognostic features of plasmacytomas: a multicenter study of Turkish Oncology Group-Sarcoma Working Party. Am J Hematol 2008;83:702-7. [Crossref] [PubMed]
- Reed V, Shah J, Medeiros LJ, et al. Solitary plasmacytomas: outcome and prognostic factors after definitive radiation therapy. Cancer 2011;117:4468-74. [Crossref] [PubMed]
- Suh YG, Suh CO, Kim JS, et al. Radiotherapy for solitary plasmacytoma of bone and soft tissue: outcomes and prognostic factors. Ann Hematol 2012;91:1785-93. [Crossref] [PubMed]
- Avilés A, Huerta-Guzmán J, Delgado S, et al. Improved outcome in solitary bone plasmacytomata with combined therapy. Hematol Oncol 1996;14:111-7. [Crossref] [PubMed]
- Mayr NA, Wen BC, Hussey DH, et al. The role of radiation therapy in the treatment of solitary plasmacytomas. Radiother Oncol 1990;17:293-303. [Crossref] [PubMed]
Cite this article as: Chen HL, Chou YH, Lee YC, Tseng HC. Solitary plasmacytoma of bone over sacrum: a case report. Ther Radiol Oncol 2019;3:8.