
Stereotactic ablative radiation therapy for operable early-stage lung cancer—considerations and controversies
SABR in early-stage non-small cell lung cancer (NSCLC)
Stereotactic ablative radiation therapy (SABR) for the treatment of early-stage NSCLC is a precise and safe radiation therapy technique (Figure 1) that has been available since the 1990s (1). It offers local tumour control rates greater than 90% at 5 years as demonstrated in the recently updated results of the RTOG 0236 study that was initially activated in the early 2000s (2). A pair of randomized studies that compared SABR to protracted conventionally fractionated radiotherapy in medically inoperable patients have been recently reported. These include the randomized SPACE trial (n=102), which prescribed 15 Gy ×3 to the periphery of the planning target volume (PTV) in the cohort randomized to SABR. It was the first to complete and demonstrated that when compared to conventional long-course radiotherapy, patients treated with SABR had less dyspnea, chest pain, or cough; although, local control and overall survival (OS) rates were equivalent (3). Meanwhile, in initial findings from the randomized CHISEL trial (n=101), which prescribed 18 Gy ×3 or 12 Gy ×4 to the periphery of the PTV in the cohort randomized to SABR, patients were found to have superior local control and OS rates when compared to the longer course of protracted conventional radiotherapy (4).
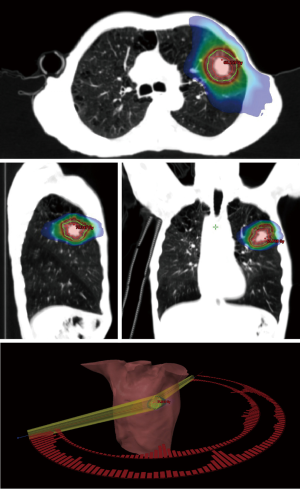
Today, SABR is considered the standard of care for patients with stage I NSCLC whenever they are inoperable, or decline surgery (4,5). Yet, when delivered to a select series of operable patients, the results of SABR are surprisingly comparable to prospective surgical outcomes. Multiple prospective and retrospective series have now shown that whenever SABR is delivered to patients with a longer life expectancy, OS rates can be 77–95% (6-12) and 45–70% (8,10,13-15), at 3 and 5 years, respectively (Table 1). A deeper analysis of these operable series demonstrates local control rates between 86–97% can be achieved at 5 years (10,13-15) (Table 1), which once again represents results that are comparable with long-term surgical data (18,19). Such reports have led many to question the primacy of surgery over radiotherapy as a curative treatment for lung cancer, and continue to provide the foundation for multiple phase III trials that have attempted to compare these two treatments in a randomized fashion (NCT00687986, NCT00840749, NCT01336894).
Table 1
| Study | Year | Study design | Dose/fraction | Size (n) | Age, median [range] (years) | 3-y results | 4-y results | 5-y results | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS, % | PFS, % | OS, % | PFS, % | LC, % | OS, % | PFS, % | CSS, % | LC, % | ||||||||
| Uematsu |
2001 | Retrospective | 50–60 Gy/5–10# | 29 | 71 [54–86] | 86 | – | – | – | – | – | – | – | – | ||
| Chang |
2015 | Prospective | 54 Gy/3#; 50 Gy/4#; 60 Gy/5# | 31 | 67.1 [43–82] | 95 | 96 | – | – | – | – | – | – | – | ||
| Komiyama |
2015 | Retrospective | 32–70 Gy/4–15# | 661 | 75 | – | – | 79 | – | – | – | – | – | – | ||
| Timmerman |
2013, 2018 | Prospective | 54 Gy/3# | 26 | 72.5 [54–88] | – | – | 57 | 56 | 96 | – | – | – | – | ||
| Lagerwaard |
2012 | Retrospective | 60 Gy/3#; 60 Gy/5#; 60 Gy/8# | 177 | 76 [50–91] | 84.7 | 81 | – | – | – | 51.3 | – | – | – | ||
| Onishi |
2011 | Retrospective | 45–72.5 Gy/3–10# | 87 | 74 | – | – | – | – | – | 69.5 | – | 76.1 | 86.7 | ||
| Shibamoto |
2015 | Prospective | 44 Gy/4#; 48 Gy/4#; 52 Gy/4# | 60 | 77 [29–89]‡ | – | – | – | – | – | 66 | – | 74 | 88 | ||
| Nagata |
2015, 2018 | Prospective | 48 Gy/4# | 64 (3 y); 40 (5 y) | 79§ | 76.5 | 54.5 | – | – | – | 54 | – | – | 85.4¶ | ||
| Eriguchi |
2017 | Retrospective | 40 Gy/5#; 50 Gy/5#; 60 Gy/5# | 88 | 79 [55–88] | 86 | – | – | – | – | 69 | – | 88 | 93 | ||
| Schonewolf |
2018 | Retrospective | BED ≥100 Gy10 | 34 | 73 [55–92] | – | – | – | – | – | 45.3 | 82.4 | 91 | 96.7 | ||
†, medically operable subgroup; ‡, ages for entire cohort (ages for medically operable subgroup not given); §, significant difference between SABR arm median age (79 years) and lobectomy arm median age (62 years), P<0.001; ¶, 10-y LC% remained at 85.4%, 10-y OS was 23.8% (
Potential advantages of SABR over surgery for stage I NSCLC
Supported by the above efficacy data, the utilization of SABR for early NSCLC has continued to grow. A recently published analysis from the Surveillance, Epidemiology and End Results (SEER) database has shown an increase in utilization of definitive radiotherapy for stage I NSCLC from 13% to 29% between 2004 and 2012 that is coincident with a rise in the popularity with SABR (20). What factors may be driving physician and patient uptake of SABR? We believe that when applied to carefully selected patients, SABR presents a more attractive treatment proposition for patients and physicians when compared to an operation that requires a hospitalization that may result in a prolonged stay or complication. It is a convenient and safe outpatient procedure that does not expose patients to the known risks of early or delayed postoperative morbidity or mortality that can be seen following pulmonary resections when performed at low-volume hospitals by surgeons who are not specialized in thoracic surgery (21-24). Whilst it is true that very low 90-day postoperative mortality rates for stage I lung cancer of approximately 1–2% have been achieved in select series (25), these data come from specialized high volume thoracic surgical centres, and do not necessarily reflect outcomes in the general community. For example, in the UK National Lung Cancer Audit, 30-day mortality following surgery for stage IA NSCLC was 1.6%, rising to 3.4% by 90 days, and for stage IB 30-day mortality was 2.8%, rising to 5.5% by 90 days (26). In that same report, mortality rates increased with advancing age for all stages, with a 90-day mortality rate of 7.3% in patients aged 70–74 rising to 16.5% in patients aged 85 years or older (26). These increased 90-day mortality data are broadly consistent with other published surgical outcomes series, and have been summarized previously (27). In a similar fashion, a retrospective analysis of over 2,000 patients with stage I NSCLC from a high-volume academic hospital in North America showed that the non-cancer cumulative incidence of death after surgery was higher than cancer-related deaths until 1.5 years post-operatively, and in patients older than 75 years, this period was 2.5 years (25). This phenomenon of increased early mortality in patients undergoing thoracic surgery over radiotherapy has been coined “the head-start effect” (28), and given the efficacy of SABR now raises the question whether or not it is ethical to operate on patients who are at risk for premature mortality for treatment of an asymptomatic stage I NSCLC.
SABR without biopsy confirmation
Although it is demonstrably effective, there are several controversies in the application of SABR to patients with stage I NSCLC, whether patients are operable or not. A particular scenario in which SABR is sometimes recommended—and appropriately criticised—is in patients with unbiopsied lung lesions that carry a high clinical suspicion for malignancy. This naturally creates the potential for futile therapy in an empiric setting, unnecessary exposure to radiation-related toxicities, and may contribute to overestimations in the efficacy of SABR in patients with benign lung nodules. In patients with benign nodules it may also lead to a scenario where a patient has a complicated region of SABR-related fibrosis that requires additional invasive procedures in someone who never had lung cancer in the first place (29).
While the authors of this review believe that the first preference should be to always obtain a pre-treatment tissue diagnosis before SABR, there are numerous situations where this is not possible due to patient or technical factors, including lack of advanced endobronchial equipment or expertise. This dilemma can affect patients who are considered for either surgery or SABR. Recently published data inform us that the rate of benign disease at the time of pulmonary resection—where pre-operative biopsy was not performed—ranges from 11% to 20% (30,31). This includes a recent report from the Cancer and Leukaemia Group B (CALGB) 140503 trial of lobectomy versus sublobar resection trial that found the benign nodule rate at surgery was approximately 20% (32). While studies like these measure the incidence of futile surgery, and provide insights into the rate of potentially futile SABR, they might actually present an argument in favour of empiric SABR over a surgical biopsy given its lower risk of treatment-related complications. For example, in the NELSON lung cancer screening trial which reported a rate of minor and major complications after thoracotomy in 47% and 10% respectively, approximately 1 in 5 of these complications occurred following operations for benign disease which would have likely been avoided with empiric SABR (33).
Fortunately, there are nomograms that can predict the probability of malignancy when considering age, smoking history, and radiographic features; though there will always be times of uncertainty. As such, a threshold of 85% risk of malignancy to proceed with an intervention has been suggested as an appropriate level that achieves a balance between the risk of toxicity from an unnecessary procedure versus risk of untreated tumour progression (34); although, this same cut-off may not be appropriate in regions with a high risk of benign granulomatous disease. While discussions about surgical resection to satisfy a requirement for diagnosis have been largely unchallenged in the past, there are now many scenarios where the alternative option of empiric SABR might be more appropriate. As we have alluded to, these scenarios are quite complex, and warrant multidisciplinary discussions, especially if the risk of a surgical complication is high.
SABR without mediastinal staging
In a similar vein to concerns about irradiation of unbiopsied primary lesions, the lack of pathologic mediastinal staging with SABR has also attracted criticisms out of concerns that patients may miss out on the opportunity for life-prolonging adjuvant chemotherapy in those who would be upstaged at the time of surgery. This is even though the value of optimizing the discovery of occult mediastinal disease was unable to be measured in the American College of Surgeons Oncology Group (ACOSOG) Z0030 trial which randomized over 1,000 patients between mediastinal sampling vs. dissection and found no improvement in OS with a more thorough staging process (19). It is known from the lung adjuvant cisplatin evaluation (LACE) meta-analysis that there can be a 5% OS advantage with the addition of adjuvant chemotherapy in patients who are upstaged to pathologic stage II–III NSCLC after pulmonary resection and surgical lymph node staging (35). However, as Louie et al. have illustrated, even when assuming a 15% occult nodal metastasis rate, and an estimation of only 66% receiving guideline directed adjuvant chemotherapy, only 0.5 lives are prolonged for every 100 patients who undergo surgical staging of the mediastinum—a benefit that would be abrogated if the surgical mortality exceeded 0.5% (34). For now, it remains unclear why isolated nodal failures are rare in the context of SABR (36), though we postulate that incidental low-dose irradiation of mediastinal nodes, or potential immune activation and eradication of nodal deposits, are possible explanations that require further supporting evidence.
Long-term outcomes with SABR
An additional controversy about the idea of SABR in operable patients relates to concerns about the paucity of long-term tumour control rates, as many initial reports were limited to only 3 years of follow-up (37). However, as is presented in Table 1, contemporary series of operable patients have now reported up to 5-year data, with local tumour control rates in the range of 86–96% with SABR. In the long-awaited update from the RTOG 0236 study, additional cancer recurrences after 5 years were particularly found to occur in untreated locations in the chest (2).
Management of relapse following SABR
The management of patients with local or regional failure after SABR presents a challenge that is best addressed through a multidisciplinary approach. This is because patients are often still curable, as was recently demonstrated in a large series from MD Anderson that reported the outcomes for the 11.2% of patients who developed non-metastatic relapse following upfront SABR (38). Patients were managed with a variety of salvage strategies including repeat SABR, surgery, thermal ablation, chemotherapy, chemoradiotherapy, conventional radiotherapy, and even brachytherapy, though some patients did not receive any further treatment. Interestingly, the authors reported that patients who received salvage therapies for an isolated local relapse had similar survival when compared to those without recurrence. Survival was poorer for patients with isolated regional relapse, though their outcomes were similar to patients with stage III disease. Finally, survival was poorest for those who did not receive any salvage therapies.
With regard to the safety of salvage surgery after SABR, there are now multiple reports in the literature, including the use of minimally invasive resections with mediastinal sampling (39). In the largest series reported to date, Antonoff et al. detailed the outcomes of a selected series of 37 patients who underwent salvage surgery for an isolated local failure at a median of 16.2 months after SABR. Approximately half of the resections did not report any extensive adhesions, and negative margins were obtained in 100% (40). They reported a perioperative mortality rate of 0% related to previous SABR, and the 3-year OS was 71% which provides further support that a strategy of upfront SABR with reservation of surgery for treatment failure might be a viable strategy to investigate in prospective clinical trials. Particularly, as this is a treatment paradigm that has been adopted for routine oncological care for malignant tumours of the head and neck, cervix, and anal canal with good effect. It offers a favourable early toxicity profile of radiation therapy in the first instance, acknowledging that even a slightly inferior local control rate does not confer inferior survival because of the opportunity for surgical salvage intervention(s). Such a management strategy for stage I NSCLC is now inherently being tested in the ongoing randomized trials of surgery vs. SABR for operable patients who are likely to remain operable at time of relapse (NCT02984761, NCT02468024, NCT01753414).
The trials and tribulations of comparing available data
While we await the completion of randomized trials, we are left with retrospective comparisons that analyse datasets of convenience with statistical techniques, such as propensity score matching, that aim to reduce bias in non-randomized data. It is worth considering that such approaches might not actually achieve this aim, and so retrospective studies that used this approach should be interpreted with caution (41). That is because for any such comparisons to be meaningful, the data must first be established on a level playing field, without inherent differences in the life expectancy of each patient group, and assurances that interpretations of the analyses are devoid of specialty bias. This unfortunately cannot be achieved with a high degree of integrity given currently available retrospective analyses are confounded as a result of over 80 years of history that has marked surgical resection as the standard of care, with reservation of radiotherapy only for those who are frail, elderly, or have other conditions that deem them unsafe for surgery (42). By way of example, a recently published propensity matched retrospective analysis compared the outcomes of patients selected for video-assisted thoracic surgery (VATS) lobectomy or SABR for stage I NSCLC, and found a significantly higher rate of survival at 3 years among the group of patients who were offered surgery (43). But as Stokes and Rusthoven wrote in a related editorial, these data were “confounded by operability” which introduced limitations into modelling efforts to match the groups as 70% of patients in this propensity-matched SABR group were deemed medically inoperable, compared with 0% (by definition) in the surgical arm (44). There are now over two dozen similar publications, despite calls from editors to preserve caution about specialty bias whenever interpreting studies like these (45). Particularly as a recent meta-analysis of propensity score studies demonstrated that the first author specialty (thoracic surgery or radiation oncology) was one of the strongest predictors of survival in early lung cancer (46).
Another challenge with interpreting the retrospective literature concerns the assessment of local failure after SABR because of the scarring effects of radiation on normal lung tissue which can simulate or mask recurrence, making interpretation of imaging difficult. Radiographic findings predictive of local recurrence after SABR have been proposed, and include features such as cranio-caudal growth, serial enlargement, loss of air bronchogram and loss of linearity (47,48), but these are admittedly imperfect. Following surgery, definitions of local failure vary between published reports, and vary depending upon whether a lobectomy or sublobar resection was performed, and the rate of reporting of local failure can vary, depending on the reporting strategy used (18).
For now, the sole prospective randomized evidence we have available to compare SABR and surgery for early-stage operable lung cancer is the controversial pooled analysis of the STARS and ROSEL trials, which combined the results of two phase III trials (n=58) that closed early due to poor accrual. Another phase III prospective trial, ACOSOG Z4099/RTOG 1021 (49), also closed early after enrolling only ten patients, and did not publish their results. The STARS-ROSEL pooled data were encouraging for SABR, showing a 3-year OS of 95% in that group with a median follow-up of 40 months; this OS result was 15% higher than the surgical group (9). However, this publication was appropriately criticised because of the higher than expected mortality in the surgical group, and high likelihood of a spurious finding given more than 1,400 additional patients needed still to be randomized (50). Notwithstanding the unexpected magnitude of the disparity in survival outcomes, the shape of the survival curves (parallel after 18 months following an initial postoperative decline in the surgical arm) is illustrative of the aforementioned head-start effect, in describing a survival benefit afforded to SABR, assuming an equivalent or near equivalent oncologic effect, simply due to the avoidance of acute and delayed postoperative mortality (28). The remarkable results in patients who received SABR in this series remain compelling, and invite speculation as to what findings might emerge in a larger well-powered randomized controlled trial (RCT) with better surgical outcomes in the comparator arm.
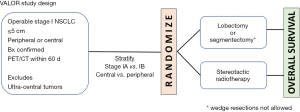
Today, there are three prospective randomized controlled trials ongoing that are comparing SABR vs. surgery for patients with operable early NSCLC (51-53). The VALOR trial (NCT02984761) (Figure 2) is open to patients with either peripheral or central biopsy-confirmed tumours up to 5 cm, and presents patients the opportunity to randomly receive either SABR or an anatomic resection (lobectomy or segmentectomy with mandatory lymph node sampling) with a primary outcome measure of 5-year OS (51). The STABLE-MATES (NCT02468024) trial is enrolling high-risk operable patients with tumours up to 4 cm who are unable to tolerate lobectomy, and offers either SABR or a sublobar resection via randomized allocation or patient preference; wedge resections require a 1 cm margin, and lymph node sampling is recommended but not required, with a primary outcome of 3-year OS (52). The POSTILV (NCT01753414) trial is limited to patients with tumours <3 cm and randomly allocates them to either SABR or sublobar resection with a 2cm margin, with mandatory nodal sampling and a primary outcome of 2-year local control (53). Each of these studies provide a meaningful opportunity to have better balanced groups for comparisons that are meaningful, such that differences between treatments may be more reliably compared.
Lessons learned from challenges with recruitment to closed randomized trials
Key lessons were learned from the failures of previous RCT trials of SABR versus surgical resection. While many of the obstacles may have been clear, clinicians and recruiters invariably had hidden biases that remained unrecognized until it was too late. This includes various forms of bias that are difficult to hide, which can emerge through covert or subliminal manifestations during the recruitment process (54). They were found to relate primarily to the challenges of maintaining equipoise, managing patient preferences for more or less invasive treatments, and the difficulties in educating patients about the importance of accepting a randomly allocated treatment (55). As with other similar trials of surgery versus radiotherapy, research staff were found to retreat prematurely during discussions about enrolment upon discovering patient preferences, even though such a preference may be uninformed and openly uncertain (56,57).
These challenges are not new in medicine, and have been addressed with various strategies in the past. One such approach, known as “pre-randomization”, was pioneered by Marvin Zelen in 1979 and ultimately helped investigators complete the landmark National Surgical Adjuvant Breast and Bowel Project (NSABP)-B06 randomized clinical trial of total mastectomy versus segmental mastectomy with whole breast radiotherapy; a trial which had initially struggled to accrue. This approach presents patients who are eligible with only one of the treatments, and offers them the opportunity to enroll in the trial only if they accept the treatment. This is now in use in the STABLE-MATES trial, though patients who refuse this pre-randomized treatment allocation also have the option to choose their own treatment instead and be followed in a separate cohort; it’s believed since patients are eligible for surgery, even patients in this self-selected cohort are more likely to be balanced when compared to retrospective studies.
Insights into the future
As the above randomized studies continue to accrue, and may some day present final results, it deserves considering how the outcomes may influence practice in the future. Even if an OS advantage is demonstrated for patients treated with upfront SABR, it is inevitable that subgroups of operable patients will be better managed with upfront surgery instead. For now, the enthusiasm for SABR is raising the bar for thoracic surgeons to consider more thoughtful patient selection, minimally invasive resections, and surgical nodal staging. Yet, once published, there are three different scenarios to envision: (I) if the OS rate is superior with SABR, then it is likely that practice guidelines will promote SABR as the standard of care in early lung cancer, (II) if the OS rates are similar, then clinicians will more thoroughly consider secondary conditions such as surgical fitness and patient preferences, and engage in complex shared decisions about an optimal treatment decision for any given patient, and (III) if SABR is found to provide a lower probability of long-term survival, then we will for the first time have level I evidence that the risks of a pulmonary resection are justified if long-term survival beyond a few years is an important goal of care.
While we predict a day will come when adequately powered phase III data are available, it deserves emphasis that the history of medicine has shown us that clinical practice patterns can be slow to change even years after randomized evidence are published (58). However, considering the referral pathways of patients with radiographic evidence of stage I NSCLC, the influencers for this population will not be limited to any single specialty, and will need to consider pulmonologists, interventional radiologists, and primary care physicians who typically see these patients well before either of the treating physicians.
Conclusions
SABR is demonstrably effective and safe in controlling early NSCLC in inoperable patients. The limited available data for operable patients out to 5 years after treatment are compelling but incomplete. As such, they do not currently justify routinely offering this treatment as an alternative to surgical resection, which remains an appropriate treatment to prefer in the first instance. Despite some controversy, the increasing use of SABR in the operable setting suggests that patients and clinicians have started to challenge the current early NSCLC treatment paradigm in the absence of prospectively gathered evidence, so there is an imperative to test surgery and SABR prospectively in operable patients whilst clinical equipoise exists. There are indeed cultural challenges to this endeavour, but these are not insurmountable, so we keenly await the availability of prospectively gathered data. In the interim we would do well to keep in mind that even if a survival benefit is shown with SABR, there will undoubtedly be cases in which a surgical approach is preferred. Accordingly, we recommend treatment as per published guidelines and encourage the referral of suitable patients for STABLE-MATES, VALOR and POSTILV clinical trial participation.
Acknowledgments
Jenny Donovan for her contribution to the discussion of challenges in recruitment to randomized controlled trials.
Funding: S Siva is supported by a National Health and Medical Research Council Early Career Fellowship APP1122347 and a Peter MacCallum Cancer Centre Clinical Fellowship.
Footnote
Conflicts of Interest: S Siva is the recipient of a grant from Varian Medical systems for research related to SABR in kidney cancer. D Moghanaki is employed by the Department of Veterans Affairs and receives support from the Veterans Affairs Cooperative Studies Program. He has received travel reimbursement and speaking honoraria from Varian Medical Systems. Dr. CP Daniels has no conflicts of interest to declare.
Disclaimer: The views expressed in this article do not necessarily represent those of the Department of Veterans Affairs, or the U.S. government.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Blomgren H, Lax I, Näslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995;34:861-70. [Crossref] [PubMed]
- Timmerman RD, Hu C, Michalski JM, et al. Long-term Results of Stereotactic Body Radiation Therapy in Medically Inoperable Stage I Non-Small Cell Lung Cancer. JAMA Oncol 2018;4:1287-8. [Crossref] [PubMed]
- Nyman J, Hallqvist A, Lund JÅ, et al. SPACE - A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol 2016;121:1-8. [Crossref] [PubMed]
- Ball D, Mai T, Vinod S, et al. MA 13.07 A Randomized Trial of SABR vs Conventional Radiotherapy for Inoperable Stage I Non-Small Cell Lung Cancer: TROG09.02 (CHISEL). J Thorac Oncol 2017;12:S1853. [Crossref]
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 2.2019) [Internet]. NCCN Clinical Practice Guidelines in Oncology. 2018 [cited 2018 Dec 6]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Timmerman RD, Paulus R, Pass HI, et al. Stereotactic body radiation therapy for operable early-stage lung cancer: Findings from the NRG oncology RTOG 0618 trial. JAMA Oncol 2018;4:1263-6. [Crossref] [PubMed]
- Eba J, Nakamura K, Mizusawa J, et al. Stereotactic body radiotherapy versus lobectomy for operable clinical stage IA lung adenocarcinoma: Comparison of survival outcomes in two clinical trials with propensity score analysis (JCOG1313-A). Jpn J Clin Oncol 2016;46:748-53. [Crossref] [PubMed]
- Nagata Y, Hiraoka M, Shibata T, et al. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys 2015;93:989-96. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Eriguchi T, Takeda A, Sanuki N, et al. Stereotactic body radiotherapy for operable early-stage non-small cell lung cancer. Lung Cancer 2017;109:62-7. [Crossref] [PubMed]
- Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:348-53. [Crossref] [PubMed]
- Uematsu M, Shioda A, Suda A, et al. Computed tomography-guided frameless stereotactic radiotherapy for stage I non-small cell lung cancer: a 5-year experience. Int J Radiat Oncol Biol Phys 2001;51:666-70. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable Stage I non-small-cell lung cancer: Can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011;81:1352-8. [Crossref] [PubMed]
- Schonewolf CA, Heskel M, Doucette A, et al. Five-year Long-term Outcomes of Stereotactic Body Radiation Therapy for Operable Versus Medically Inoperable Stage I Non-small-cell Lung Cancer: Analysis by Operability, Fractionation Regimen, Tumor Size, and Tumor Location. Clin Lung Cancer 2019;20:e63-71. [Crossref] [PubMed]
- Shibamoto Y, Hashizume C, Baba F, et al. Stereotactic body radiotherapy using a radiobiology-based regimen for Stage I non-small-cell lung cancer: Five-year mature results. J Thorac Oncol 2015;10:960-4. [Crossref] [PubMed]
- Komiyama T, Onishi H, Shioyama Y, et al. ORAL19.05 Japanese Multicenter Study of Stereotactic Body Radiotherapy for 661 Medically Operable Patients with Stage I Non-Small Cell Lung Cancer. J Thorac Oncol 2015;10:S173-260.
- Nagata Y, Hiraoka M, Shibata T, et al. A phase II trial of stereotactic body radiation therapy for operable T1N0M0 non-small cell lung cancer: Japan Clinical Oncology Group (JCOG0403)—Long term follow-up results. J Clin Oncol 2018;36:8512. [Crossref]
- Fedor D, Rainey Johnson W, Singhal S. Local recurrence following lung cancer surgery: Incidence, risk factors, and outcomes. Surg Oncol 2013;22:156-61. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: Results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Haque W, Szeja S, Tann A, et al. Changes in Treatment Patterns and Overall Survival in Patients With Early-Stage Non-Small Cell Lung Cancer in the United States After the Incorporation of Stereotactic Ablative Radiation Therapy: A Population-based Analysis. Am J Clin Oncol 2018;41:259-66. [PubMed]
- Farjah F, Flum DR, Varghese TK Jr, et al. Surgeon Specialty and Long-Term Survival After Pulmonary Resection for Lung Cancer. Ann Thorac Surg 2009;87:995-1004; discussion 1005-6. [Crossref] [PubMed]
- Schipper PH, Diggs BS, Ungerleider RM, et al. The Influence of Surgeon Specialty on Outcomes in General Thoracic Surgery: A National Sample 1996 to 2005. Ann Thorac Surg 2009;88:1566-72; discussion 1572-3. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-e313S.
- Goodney PP, Lucas FL, Stukel TA, et al. Surgeon Specialty and Operative Mortality with Lung Resection. Ann Surg 2005;241:179-84. [PubMed]
- Eguchi T, Bains S, Lee MC, et al. Impact of Increasing Age on Cause-Specific Mortality and Morbidity in Patients With Stage I Non-Small-Cell Lung Cancer: A Competing Risks Analysis. J Clin Oncol 2017;35:281-90. [Crossref] [PubMed]
- Powell HA, Tata LJ, Baldwin DR, et al. Early mortality after surgical resection for lung cancer: An analysis of the English National Lung cancer audit. Thorax 2013;68:826-34. [Crossref] [PubMed]
- Senthi S, Senan S. Surgery for early-stage lung cancer: Post-operative 30-day versus 90-day mortality and patient-centred care. Eur J Cancer 2014;50:675-7. [Crossref] [PubMed]
- Rusthoven CG, Palma DA, Senan S, et al. The Head Start Effect: Will Acute and Delayed Postoperative Mortality Lead to Improved Survival with Stereotactic Body Radiation Therapy for Operable Stage I Non – Small-Cell Lung Cancer? J Clin Oncol 2017;35:1749-51. [Crossref] [PubMed]
- Ronden MI, van Sörnsen de Koste JR, Johnson C, et al. Incidence of High-Risk Radiologic Features in Patients Without Local Recurrence After Stereotactic Ablative Radiation Therapy for Early-Stage Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2018;100:115-21. [Crossref] [PubMed]
- Flores R, Bauer T, Aye R, et al. Balancing curability and unnecessary surgery in the context of computed tomography screening for lung cancer. J Thorac Cardiovasc Surg 2014;147:1619-26. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Kohman LJ, Gu L, Altorki N, et al. Biopsy first: Lessons learned from CALGB 140503. J Thorac Cardiovasc Surg 2017;153:1592-7. [Crossref] [PubMed]
- Van't Westeinde SC, Horeweg N, De Leyn P, et al. Complications following lung surgery in the Dutch-Belgian randomized lung cancer screening trial. Eur J Cardiothorac Surg 2012;42:420-9. [Crossref] [PubMed]
- Louie AV, Senan S, Patel P, et al. When is a biopsy-proven diagnosis necessary before stereotactic ablative radiotherapy for lung cancer?: A decision analysis. Chest 2014;146:1021-8. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Ward MC, Oh SC, Pham YD, et al. Isolated Nodal Failure After Stereotactic Body Radiotherapy for Lung Cancer: The Role for Salvage Mediastinal Radiotherapy. J Thorac Oncol 2016;11:1558-64. [Crossref] [PubMed]
- Weder W, Moghanaki D, Stiles B, et al. The great debate flashes: Surgery versus stereotactic body radiotherapy as the primary treatment of early-stage lung cancer. Eur J Cardiothorac Surg 2017;53:295-305. [Crossref] [PubMed]
- Brooks ED, Sun B, Feng L, et al. Association of long-term outcomes and survival with multidisciplinary salvage treatment for local and regional recurrence after stereotactic ablative radiotherapy for early-stage lung cancer. JAMA Netw Open 2018;1:e181390 [Crossref]
- Van Breussegem A, Hendriks JM, Lauwers P, et al. Salvage surgery after high-dose radiotherapy. J Thorac Dis 2017;9:S193-200. [Crossref] [PubMed]
- Antonoff MB, Correa AM, Sepesi B, et al. Salvage pulmonary resection after stereotactic body radiotherapy: A feasible and safe option for local failure in selected patients. J Thorac Cardiovasc Surg 2017;154:689-99. [Crossref] [PubMed]
- King G, Nielsen R. Why Propensity Scores Should Not Be Used for Matching | GARY KING [Internet]. 2016 [cited 2018 Dec 6]. Available online: https://gking.harvard.edu/publications/why-propensity-scores-should-not-be-used-formatching
- Moghanaki D, Chang JY. Is surgery still the optimal treatment for stage I non-small cell lung cancer? Transl Lung Cancer Res 2016;5:183-9. [Crossref] [PubMed]
- Cornwell LD, Echeverria AE, Samuelian J, et al. Video-assisted thoracoscopic lobectomy is associated with greater recurrence-free survival than stereotactic body radiotherapy for clinical stage I lung cancer. J Thorac Cardiovasc Surg 2018;155:395-402. [Crossref] [PubMed]
- Stokes WA, Rusthoven CG. Surgery vs. SBRT in retrospective analyses: confounding by operability is the elephant in the room. J Thorac Dis 2018;10:S2007-10. [Crossref] [PubMed]
- Kidane B. Stereotactic body radiation therapy versus video-assisted thoracoscopic surgery in stage I lung cancer: Honesty in the face of uncertainty. J Thorac Cardiovasc Surg 2018;155:365-6. [Crossref] [PubMed]
- Chen H, Laba JM, Boldt RG, et al. Stereotactic Ablative Radiation Therapy Versus Surgery in Early Lung Cancer: A Meta-analysis of Propensity Score Studies. Int J Radiat Oncol Biol Phys 2018;101:186-94. [Crossref] [PubMed]
- Huang K, Dahele M, Senan S, et al. Radiographic changes after lung stereotactic ablative radiotherapy (SABR)--can we distinguish recurrence from fibrosis? A systematic review of the literature. Radiother Oncol 2012;102:335-42. [Crossref] [PubMed]
- Ronden MI, Palma D, Slotman BJ, et al. Brief Report on Radiological Changes following Stereotactic Ablative Radiotherapy (SABR) for Early-Stage Lung Tumors: A Pictorial Essay. J Thorac Oncol 2018;13:855-62. [Crossref] [PubMed]
- Fernando HC, Timmerman R. American College of Surgeons Oncology Group Z4099/Radiation Therapy Oncology Group 1021: a randomized study of sublobar resection compared with stereotactic body radiotherapy for high-risk stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2012;144:S35-8. [Crossref] [PubMed]
- Samson P, Keogan K, Crabtree T, et al. Interpreting Survival Data from Clinical Trials of Surgery versus Stereotactic Body Radiation Therapy in Operable Stage I Non-Small Cell Lung Cancer Patients. Lung Cancer 2017;103:6-10. [Crossref] [PubMed]
- Veterans Affairs Lung Cancer Or Stereotactic Radiotherapy (VALOR). NCT02984761 [Internet]. [cited 2018 Nov 18]. Available online: https://clinicaltrials.gov/ct2/show/record/NCT02984761
- JoLT-Ca Sublobar Resection (SR) Versus Stereotactic Ablative Radiotherapy (SAbR) for Lung Cancer (STABLE-MATES). NCT02468024 [Internet]. [cited 2018 Nov 18]. Available online: https://clinicaltrials.gov/ct2/show/NCT02468024
- Radical Resection Vs. Ablative Stereotactic Radiotherapy in Patients With Operable Stage I NSCLC (POSTILV). NCT01753414 [Internet]. [cited 2018 Nov 18]. Available online: https://clinicaltrials.gov/ct2/show/NCT01753414
- Rooshenas L, Elliott D, Wade J, et al. Conveying Equipoise during Recruitment for Clinical Trials: Qualitative Synthesis of Clinicians' Practices across Six Randomised Controlled Trials. PLoS Med 2016;13:e1002147 [Crossref] [PubMed]
- Jepson M, Elliott D, Conefrey C, et al. An observational study showed that explaining randomization using gambling-related metaphors and computer-agency descriptions impeded randomized clinical trial recruitment. J Clin Epidemiol 2018;99:75-83. [Crossref] [PubMed]
- Mills N, Blazeby JM, Hamdy FC, et al. Training recruiters to randomized trials to facilitate recruitment and informed consent by exploring patients' treatment preferences. Trials 2014;15:323. [Crossref] [PubMed]
- Mills N, Donovan JL, Wade J, et al. Exploring treatment preferences facilitated recruitment to randomized controlled trials. J Clin Epidemiol 2011;64:1127-36. [Crossref] [PubMed]
- Lammers A, Mitin T, Moghanaki D, et al. Lung cancer specialists' opinions on treatment for stage I non-small cell lung cancer: A multidisciplinary survey. Adv Radiat Oncol 2018;3:125-9. [Crossref] [PubMed]
Cite this article as: Daniels CP, Moghanaki D, Siva S. Stereotactic ablative radiation therapy for operable early-stage lung cancer—considerations and controversies. Ther Radiol Oncol 2019;3:3.


