
Combining hypofractionated radiation therapy with immunotherapy for anorectal malignant melanoma: a case report
Introduction
Mucosal melanoma arises primarily in the head and neck, anorectal, and vulvovaginal regions. It is rare and account for less than 1% of all melanomas, and anorectal melanoma represents only 0.5–4% of all anorectal malignancies (1).
Because that more than half of the patients already have lymph node or distant metastases at the time of diagnosis, multimodality therapy including surgery, systemic therapy and radiation is a common approach. While surgical intervention stands for the mainstay of the primary treatment, cure is rarely achieved regarding to its aggressive natural course. Traditional chemotherapy is challenged with either low response rate or the rapid development of resistance (1). The Nobel Prize winning checkpoint inhibitor immunotherapy has established an entirely new paradigm for cancer therapy, which significantly prolongs survival in patients with metastatic melanoma (2,3). However, its role in mucosal melanoma has not been well explored (4).
Radiotherapy has been used to definitively treat unresectable malignant melanoma or to palliatively treat metastatic disease. Hypofractionated radiation therapy has been found to modulate tumor microenvironment and synergize with anticancer immunotherapy. Here we describe our experience of combining both modalities in a patient of anorectal malignant melanoma.
Case presentation
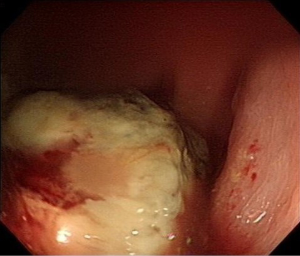
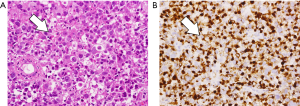
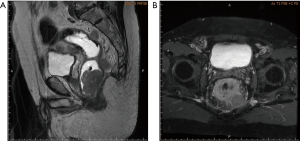
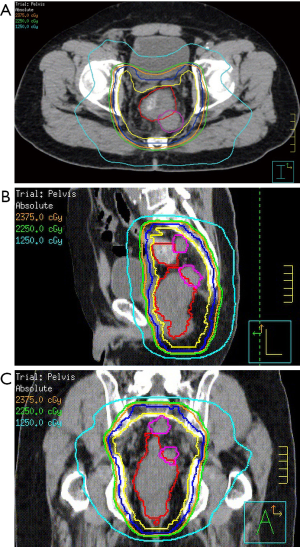
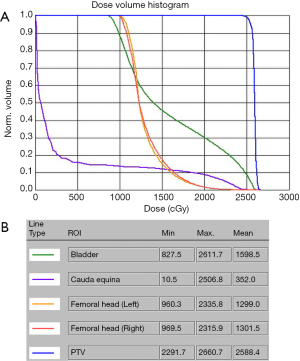
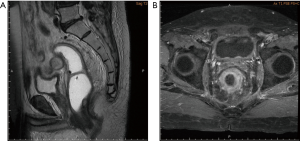
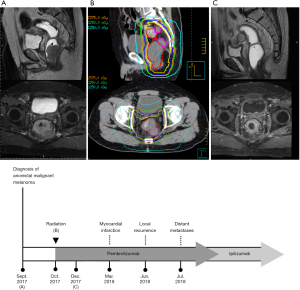
A 69-year-old vegetable vendor was admitted to our hospital after experiencing rectal bleeding and anorectal pain for several weeks. He was a non-smoker with a medical history of coronary artery disease. Digital examination and colonoscopy revealed an infiltrative mass at lower rectum (Figure 1). A biopsy was performed, and the specimens were sent for histological examination. The sections showed a picture of malignant tumor composed of ovoid epithelioid tumor cells arranged in single cell or diffuse pattern. The tumor cells had eosinophilic cytoplasm and high nucleus to cytoplasm ratio and prominent nucleoli. Frequent mitoses and focal tumor necrosis were found. By immunohistochemical stains, the tumor cells were positive for S-100 and HMB45 but negative for cytokeratin (AE1/AE3), cytokeratin 7, cytokeratin 20 and leukocyte common antigen. Malignant melanoma was diagnosed (Figure 2). No obvious melanin pigment production was found in H&E stain and Fontana Masson stain. The mutation of BRAF V600E wasn’t detected. Whole-body computed tomography and magnetic resonance imaging of the pelvis showed a 5-cm lobulated tumor with blurring margin in the posterior wall of lower rectum, accompanied with 2 loco-regional lymphadenopathies suggestive of nodal metastasis (Figure 3). No evidence of distant metastases was found. The patient was referred to our department for the disseminated anorectal malignant melanoma, stage II based on Ballantyne’s mucosal melanoma staging system (5) and stage IIIb based on AJCC 8th rectal cancer staging. After having 1 cycle of dacarbazine, he received intensity-modulated radiotherapy with a 10 MV X-ray which consisted of a 25-Gy total radiation dose in 5 fractions to primary tumor, lymphadenopathies and whole pelvis (Figures 4,5) over 5 consecutive days. One episode of CTCAE v4.0 grade 3 diarrhea for 1 month was observed after the treatment, which resolved with medications. Three weeks after the completion of radiotherapy, he started systemic therapy with pembrolizumab 150 mg once every 3 weeks. Ten weeks after the completion of radiotherapy, post-treatment imaging demonstrated near total regression of the primary lesions (Figure 6) and the primary lesions continued to shrink during regular follow-up. An episode of myocardial infarction occurred 6 months after the diagnosis and he underwent successful coronary artery stenting. Local recurrence in the rectum was biopsy proven at 9 months and peritoneal metastases was diagnosed at 10 months following initial diagnosis and radiotherapy. The patient remains alive with disease 14 months after the diagnosis and is being treated with pembrolizumab followed by ipilimumab. The disease course, radiotherapy plan and treatment response are summarized in Figure 7.
Discussion
Primary malignant melanoma of the rectum is a rare disease. It constitutes only 0.5–4% of all colorectal malignancies and less than 1% of all melanomas (1). Approximately 60% of the patients have regional lymph node involvement at presentation and 30% of patients have distant metastases at the time of diagnosis (6). The definitive diagnosis of melanoma is histopathologic. However, anorectal malignant melanoma has variable morphologies, which may lead to misdiagnosis as carcinoma or sarcoma particularly in amelanotic cases. An accurate immunohistochemical profile is crucial for diagnosis, which includes S-100 protein, Melan-A, HMB-45 and Sox10 (7). The prevalence of BRAF gene mutation is less than 10% in mucosal melanoma, and the efficacy of the target therapy with BRAF inhibitors remains unclear compares to cutaneous melanoma (8). Prognosis is generally poor with a median survival of 24 month and a 5-year survival of 10% to 20% (9). Two systems are used for staging anorectal malignant melanoma. One is made by the American Joint Commission on Cancer staging of rectal cancer, based on lymph nodes involvement and depth of primary lesion. Ballantyne’s staging system, initially devised for head and neck malignant melanoma since 1970, defines stage I with localized disease, stage II with regional lymph node involvement and stage III with distant metastases, with a median overall survival of 6.1, 3.3 and 2.8 years respectively (10). Currently there are no evidence-based clinical guidelines for this rare disease.
The mainstay of treatment for these tumors is complete surgical resection. Standard extent of surgical resection and lymph dissection is still under debate, and either wide excision, endoscopic mucosal resection, low anterior resection to an abdominoperineal resection has been reported. Since that most patients eventually developed metastases regardless of the extent of initial surgical intervention (11), perioperative morbidity and organ-function preservation should be part of the treatment consideration. With regards to chemotherapy, dacarbazine is the most widely used single agent. The response rate was 20% and the duration was 4 to 6 months (1). In addition to chemotherapy, the anti-CTLA4 immunotherapy (2) and anti-PD-1 immunotherapy (3) have been shown to significantly prolong survival in patients with metastatic melanoma. There are several retrospective studies using these agents for mucosal melanoma and the reported median progression free survival was about 4 months (4).
Malignant melanoma has traditionally been considered as a relatively radioresistant tumor. Early radiobiological studies showed a broad shoulder in cell survival curve, which indicated hypofractionated regimen may enhance treatment response (12). Accordingly, Princess Margaret Hospital experience reported a case series composed of 18 patients with mucosal malignant melanoma treated with hypofractionated radiation therapy. In this study, a fraction size of 4 Gy or larger achieved better treatment effect than that of less than 4 Gy, with 6 of 7 patients (86%) and 5 of 18 patients (28%) achieved complete remission respectively (13). Another study included 3 patients with anorectal malignant melanoma at the same hospital showed no treatment response with conventionally fractionated radiotherapy (14). From these studies, hypofractionated radiation therapy may pose radiobiological advantage over conventionally fractionated radiotherapy in treating mucosal melanoma.
Immunological mechanism may contribute to the biological effects of hypofractionated radiation therapy. Because ionizing radiation induces local inflammatory effects, it is postulated that the response to checkpoint blockade immunotherapy may be improved if it is administered in conjunction with radiotherapy in treating mucosal melanoma. The argument is consistent with findings in a case series reporting 4 patients with mucosal melanoma of lower genital tract treated by combined immunotherapy and radiotherapy (15). Complete response and stable disease were reported in 3 patients receiving 30 Gy in 5 fractions. The other patient who received 60.2 Gy in 28 fractions achieved partial response.
There is ongoing controversy on the optimal sequence of immunotherapy and radiotherapy. A study of patients with melanoma brain metastases showed that immunotherapy with anti-PDL1 and anti-CTLA4 given within 4 weeks of stereotactic radiosurgery had better response comparing to treatments given more than 4 weeks after radiosurgery (16). On the other hand, in the KEYNOTE-001 study it has been shown that survival benefit can be found in lung cancer patients treated with pembrolizumab and radiotherapy with a median interval of 9.5 months (17). Currently, there is a trend towards encouraging concurrent use or short interval sequential design if combining radiotherapy and immunotherapy (18,19). In this case, our multidisciplinary team decided to treat with immunotherapy after radiotherapy in a sequential fashion. It was only until the resolution of grade 3 diarrhea that the patient was fit enough to start pembrolizumab.
It remains unknown if elective nodal irradiation (ENI) should be considered when delineating target volumes for combined radiotherapy and immunotherapy. ENI is an effective strategy to prevent locoregional relapse in a variety of node-positive diseases in clinical radiation oncology. However, ENI attenuates tumor-specific T cells in the draining lymph nodes in preclinical models (20). The currently available hypofractionated protocol for treating rectal cancer, such as the one used in the Polish trial, involves the whole pelvic lymphatics in the target volume. Our patient presented with presacral lymphadenopathy, which suggested tumor cell presence in the pelvic lymphatics. Therefore, we adopted the Polish protocol and irradiated the whole pelvis for controlling microscopic spread. It is an open question whether immunotherapy combined with radiotherapy is sufficient to prevent out-of-field locoregional failure. More clinical evidence is keenly awaited for addressing this important question.
In short, while both radiotherapy and immunotherapy have been individually reported to effectively treat mucosal melanoma, our experience showed reasonable response of anorectal malignant melanoma to combined hypofractionated radiotherapy and immunotherapy. Data regarding to the treatment and response of anorectal malignant melanomas is limited to case reports and single-institute experiences given the overall rarity of the disease. Checkpoint blockade immunotherapy will soon be reimbursed by the National Health Insurance Administration in Taiwan for unresectable or metastatic malignant melanoma. With our experience, we urge our society to investigate the possible clinical benefits and risk profile associated with adding radiotherapy to anti-CTLA4 and anti-PD1/PDL1.
Acknowledgments
Funding: This research was supported by National Health Research Institute, Miaoli, Taiwan (grant No. NHRI-EX107-10713EI-FY1), Taipei Medical University and TMU Hospital, Taipei, Taiwan (grant No. 105TMU-CIT-02-1, 106TMU-CIT-02-1, USTP-NTUT-TMU-105-06, USTP-NTUT-TMU-106-01).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2018.12.09). JFC serve as an unpaid editorial board members of Therapeutic Radiology and Oncology from Oct 2017 to Sep 2019. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This research was approved by TMUJoint Institutional Review Board (N201803070). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Row D, Weiser MR. Anorectal melanoma. Clin Colon Rectal Surg 2009;22:120-6. [Crossref] [PubMed]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. Erratum in: N Engl J Med 2010;363:1290. [Crossref] [PubMed]
- Shoushtari AN, Munhoz RR, Kuk D, et al. The efficacy of anti‐PD‐1 agents in acral and mucosal melanoma. Cancer 2016;122:3354-62. [Crossref] [PubMed]
- Wanebo HJ, Woodruff JM, Farr GH, et al. Anorectal melanoma. Cancer 1981;47:1891-900. [Crossref] [PubMed]
- Yeh JJ, Shia J, Hwu WJ, et al. The role of abdominoperineal resection as surgical therapy for anorectal melanoma. Ann Surg 2006;244:1012-7. [Crossref] [PubMed]
- Compton LA, Murphy GF, Lian CG. Diagnostic immunohistochemistry in cutaneous neoplasia: an update. Dermatopathology (Basel) 2015;2:15-42. [Crossref] [PubMed]
- Cheng L, Lopez-Beltran A, Massari F, et al. Molecular testing for BRAF mutations to inform melanoma treatment decisions: a move toward precision medicine. Mod Pathol 2018;31:24-38. [Crossref] [PubMed]
- van Schaik PM, Ernst M, Meijer H, et al. Melanoma of the rectum: a rare entity. World J Gastroenterol 2008;14:1633-5. [Crossref] [PubMed]
- Heppt MV, Roesch A, Weide B, et al. Prognostic factors and treatment outcomes in 444 patients with mucosal melanoma. Eur J Cancer 2017;81:36-44. [Crossref] [PubMed]
- Nilsson PJ, Ragnarsson-Olding B. Importance of clear resection margins in anorectal malignant melanoma. Br J Surg 2010;97:98-103. [Crossref] [PubMed]
- Rofstad EK. Radiation biology of malignant melanoma. Acta Radiol Oncol 1986;25:1-10. [Crossref] [PubMed]
- Harwood AR, Cummings BJ. Radiotherapy for mucosal melanomas. Int J Radiat Oncol Biol Phys 1982;8:1121-6. [Crossref] [PubMed]
- Moozar KL, Wong CS, Couture J. Anorectal malignant melanoma: treatment with surgery or radiation therapy, or both. Can J Surg 2003;46:345-9. [PubMed]
- Schiavone MB, Broach V, Shoushtari AN, et al. Combined immunotherapy and radiation for treatment of mucosal melanomas of the lower genital tract. Gynecol Oncol Rep 2016;16:42-6. [Crossref] [PubMed]
- Qian JM, Yu JB, Kluger HM, et al. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer 2016;122:3051-8. [Crossref] [PubMed]
- Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895-903. [Crossref] [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [Crossref] [PubMed]
- Luke JJ, Lemons JM, Karrison TG, et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. J Clin Oncol 2018;36:1611-8. [Crossref] [PubMed]
- Marciscano AE, Ghasemzadeh A, Nirschl TR, et al. Elective Nodal Irradiation Attenuates the Combinatorial Efficacy of Stereotactic Radiation Therapy and Immunotherapy. Clin Cancer Res 2018;24:5058-71. [PubMed]
Cite this article as: Wang WJ, Lee KD, Chen WY, Chiou JF, Lu LS. Combining hypofractionated radiation therapy with immunotherapy for anorectal malignant melanoma: a case report. Ther Radiol Oncol 2019;3:1.


