
Treatment planning system and patient positioning for boron neutron capture therapy
Introduction
Boron neutron capture therapy (BNCT) has been carried out using several nuclear reactors worldwide owing to the requirement of a high-intensity neutron beam. However, this therapy has not yet become widespread, although its effectiveness has been demonstrated, because a nuclear reactor is difficult to handle for medical use and some reactors have been shut down. However, given the recent progress in accelerator and accelerator-driven-neutron source technologies, it is possible to generate a sufficient intensity of neutrons for BNCT treatment using compact accelerators, which can be installed in hospitals. Hence, it is expected that BNCT can be established as a general treatment, which can be received at a hospital, in the near future. Several development projects for compact accelerator-based neutron sources for BNCT are being undertaken around the world. In particular, in Japan, various types of commercial devices are being produced, some of which have already succeeded in generating a sufficient intensity of neutrons for BNCT treatment. Clinical trials for malignant brain tumors and head and neck cancer have been performed using the cyclotron-based BNCT device produced by Sumitomo Heavy Industry Co. (1) in order to obtain pharmaceutical approval for the device. Therefore, it is expected that BNCT will be performed in hospitals as advanced or insured medical care in the near future. However, BNCT cannot be implemented simply by producing and installing a neutron source. The treatment as a type of radiation therapy requires several peripheral devices and equipment such as a treatment planning system (TPS), patient positioning device, neutron beam monitor, and boron concentration measurement device. In reactor-based BNCT, these devices have been developed with minimal functions, and actual clinical trials are being performed by means of these devices. However, to establish BNCT as a general cancer treatment, peripheral devices in addition to the accelerator-based neutron source have to be improved and completed as medical devices.
TPS
Dose estimation in BNCT
In this section, clinical dose estimation in BNCT is explained.
Dose components for BNCT
In clinical dose estimation in BNCT, several absorbed dose components have to be computed. First, it is necessary to evaluate the “boron dose” that gives a therapeutic effect. The boron dose is an absorbed dose arising from the reaction between neutrons and the boron-10 accumulated in cells. The boron dose rate is determined by multiplying the concentration of boron-10 in each tissue and the thermal neutron flux at that point. Thus, to determine the boron dose, the values of the boron-10 concentration and thermal neutron flux at each point are both needed. Regarding the value of the boron-10 concentration, the assumed concentration values are entered for each tissue and tumor region at the treatment planning stage, which is implemented before actual irradiation (2). The actual concentration in a patient during irradiation often differs from the assumed value because it is difficult to accurately predict the concentration in the irradiation due to inter-individual variation in blood boron concentration profiles (3). However, the difference of the distribution for boron-10 around the irradiation field affects the delivery of the thermal neutrons in the field (4). Therefore, in the treatment planning stage, the values for the assumed concentration should be set to reasonable values for each tissue, based on pharmacokinetic studies (5,6), histological findings (3,7), and the results of past clinical studies. In the irradiation with the actual patient, the concentration in a blood sample obtained from a patient just before irradiation is measured by using prompt gamma-ray analysis (PGA) (8) or an inductively coupled plasma optical emission spectrometer (ICP-OES) (9,10), and the assumed values are compensated. Therefore, in the treatment planning stage, only the proper computation for the neutron flux and energy spectrum at each point is required.
Next, the “nonboron dose” has to be estimated. The nonboron dose is the absorbed dose arising from the reaction between neutrons and several atoms in the human body such as hydrogen, nitrogen, oxygen, and carbon. For dose estimation in BNCT, the “hydrogen dose” and “nitrogen dose” are important because both doses influence the total dose given to the patient. Some absorbed doses arising from reactions with the other atoms may be negligible. Further, the nonboron dose also includes the gamma-ray dose, which is composed of two components—the primary gamma-ray dose from the neutron source of the BNCT facility and the secondary gamma-ray dose generated in the human body by reaction with neutrons and hydrogen. Figure 1 shows the schema of the dose components in BNCT dosimetry. In addition, the reactions that are the basis of each absorbed dose are listed in Table 1.
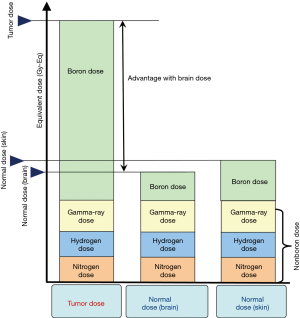
Table 1
| Dose components | Major reaction |
|---|---|
| Boron dose, DB | 10B(n,α)7Li |
| Hydrogen dose, DH | 1H(n,n)p |
| Nitrogen dose, DN | 14N(n,p)14C |
| Gamma dose, Dγ | Primary gamma dose from the beam port & Secondary gamma dose by 1H(n,γ)2H |
BNCT, boron neutron capture therapy.
For dose estimation in BNCT, all of these dose components need to be calculated. The absorbed dose Dn arising from the reactions between neutrons and each atom is determined as follows:
where f is the factor for the kinetic energy released in matter (KERMA) for neutrons or the dose conversion factor for photons and φ(t) is flux of neutrons or photons at a point. The value of f changes depending on the energy of the radiation.
Equivalent dose (ED) for BNCT
To estimate the total dose administered to a patient by neutron irradiation during therapy, the ED has to be determined. The ED for BNCT is given by
ED (Gy-Eq) = CB·DB,ppm × CBEB + DN × RBEN + DH × RBEH + Dγ
The ED is calculated by multiplying the value of the absorbed dose by the relative biological effectiveness (RBE) defined for each absorbed dose and by adding them. In Eq. [2], both RBEN and RBEH are the RBEs defined for the nitrogen and hydrogen absorbed doses, respectively, and CB is the boron-10 concentration (ppm) in each tissue. The value of the compound relative biological effectiveness (CBE) is different depending on the behavior of boron-10 compounds in each tissue. As an example of the reasons for the change in the CBE value, the difference of the cytotoxicity effect caused by the difference of the mean distance between the boron-10 accumulating in the cell and the cell nuclei affects the CBE value. The values for each RBE and CBE have been determined by evaluations based on in vivo/in vitro studies prior to clinical studies (11,12). The major RBE values applied in reactor-based BNCT are summarized in Table 2. Further, Table 3 lists the typical CBE factors for tumors and several tissues for both BPA (p-boronophenylalanine) and BSH (sodium borocaptate, Na210B12H12SH), as the boron-10 carriers applied to actual treatment of BNCT.
Table 2
| Absorbed dose | RBE value |
|---|---|
| Hydrogen dose | 2.5–3.2 |
| Nitrogen dose | 2.5–3.2 |
| Gamma dose | 1.0 |
RBE, relative biological effectiveness; BNCT, boron neutron capture therapy.
Table 3
| B-10 carriers | Brain | Skin | Mucosa | Tumor |
|---|---|---|---|---|
| BPA | 1.35 | 2.5 | 4.9 | 3.8 |
| BSH | 0.37 | 0.8 | 0.3 | 2.5 |
CBE, compound relative biological effectiveness; BPA, p-boronophenylalanine; BSH, sodium borocaptate.
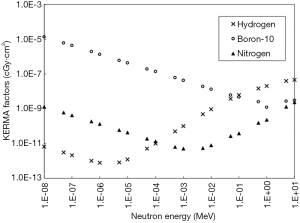
The values for the RBE, CBE, and boron-10 concentration (DB,ppm) are fixed. In treatment planning work, the user inputs the proper values. Thus, the major role of the TPS in treatment planning is to determine the distribution of each absorbed dose around the target region. From Eq. [1], the KERMA factor for neutrons and the dose conversion factor for photons employ the known values for the elements, though they change depending on the energy for both radiations. Figure 2 shows the KERMA factors for three absorbed doses. The values in the figure were extracted from the data stored in JENDL 4.0 (13) as an evaluated nuclear data. Therefore, the most important role of the TPS for BNCT is to compute the distribution of the flux of neutrons and that of photons for each energy in the estimation region precisely.
Dose calculation method
In computational dosimetry in BNCT, a Monte Carlo method—a probabilistic method—is usually used because the behavior of neutrons is complex and diverse, though the dose calculation for conventional radiotherapy such as X-ray therapy employs a deterministic method. Thus, for the TPS for BNCT, a Monte Carlo transport code is also applied to the dose calculation engine in the TPS. Most TPSs combine an external Monte Carlo code that has been developed at research institutes and is related to the nuclear field. The general-purpose Monte Carlo radiation transport code MCNP (14) has been widely applied to the dose calculation engines of some TPSs described below. This code was developed at Los Alamos National Laboratory in the USA, and it has been thoroughly validated in the nuclear field. Recently, some new TPSs have been developed in Japan, where several accelerator-based treatment devices are being developed. For these TPSs, the Particle and Heavy Ion Transport code System (PHITS) (15) as a multimodal Monte Carlo code developed by the Japan Atomic Energy Agency in Japan, is beginning to be employed. On the other hand, the “Simulation Environment for Radiotherapy Application” (SERA) (16), which is used in some BNCT facilities, combines a special Monte Carlo calculation engine.
To perform particle transport calculations with a Monte Carlo code, nuclear cross-section data that defines the radiation behavior is needed. For the particle transport calculation in the TPS, two types of representations of nuclear cross-section data are used: point-wise continuous energy cross sections and multi-group cross sections. The former cross-section data realize a high-fidelity representation of the evaluated nuclear data through the linear interpolation between a large number of specified points. In the particle transport calculation with the above external Monte Carlo codes, ENDF/B-VII (17) and JENDL 4.0 are applied as typical evaluated nuclear data for the continuous energy cross sections. In contrast to the continuous energy cross-section data, multi-group cross-section data by means of a single discrete value for the cross section over a range of particle energies achieve a more compact and computationally efficient representation, though the data are less precise. SERA employs multi-group cross-section data owing to advantages in calculation speed.
Workflow of the treatment planning for BNCT using a TPS
In the procedure for radiation therapy, it is necessary to implement treatment planning. Medical doctors decide the irradiation conditions for the patient during treatment planning prior to irradiation. The actual irradiation of a patient is implemented in accordance with the irradiation conditions. Thus, it is important to determine suitable irradiation conditions. The TPS is responsible for the treatment planning; therefore, it is one of the most important devices in radiation therapy as well as the treatment device (radiation source device). In BNCT requiring neutron irradiation, the TPS is an indispensable item.
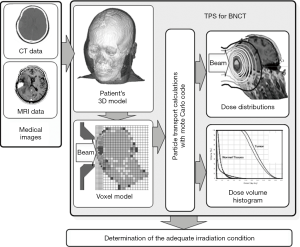
The TPS is software that can simulate the irradiation of a patient. In case of the TPS for BNCT, the system simulates the irradiation of a patient with a neutron beam, and the dose distribution around the target region is determined. The treatment planning procedure using a TPS is as follows.
- First, the patient’s medical images obtained by computed tomography (CT) and/or magnetic resonance imaging (MRI) are loaded into a TPS. The contours for some regions of interest (ROIs) for organs at risk and the gross tumor volume (GTV) or clinical target volume (CTV) as the target region are defined on each slide of the medical images.
- A patient’s three-dimensional (3D) model including the ROIs and target region is created by piling up medical images.
- The irradiation conditions including the beam incidence point, beam direction, irradiation field, energy spectrum, and intensity of the beam are set with the patient’s 3D model.
- The 3D model is converted into a voxel-based calculation model (voxel model) in order to carry out particle transport effectively.
- The distributions of both neutron and photons in the 3D model are computed by a particle transport calculation. For the calculation of the radiation distributions in BNCT dosimetry, a Monte Carlo transport algorithm is generally applied as a probabilistic method.
- The distributions of several absorbed doses, such as the boron dose, nitrogen dose, and hydrogen dose arising from the calculated radiation are determined. Several absorbed doses due to the reactions between neutrons and some atoms such as such as boron, nitrogen, and hydrogen are determined by multiplying the neutron flux and KERMA factor for each atom. For the gamma-ray dose, the dose is determined by multiplying the calculated photon flux and the dose conversion factor for a photon.
- The distributions of the radiation and several doses in the model are displayed by superimposing them on the original medical images. The minimum, maximum, and average values for the ROIs and target region are calculated. The dose-volume histogram (DVH) for each ROI is also determined.
- Different beam directions/orientations are tested to determine the optimal treatment plan. Further, by comparing the results, suitable irradiation conditions are finally determined.
Figure 3 shows the flow of the treatment planning for BNCT using a TPS.
The workflow of the TPS for BNCT is similar to other TPSs for conventional radiotherapies such as X-ray therapy and particle radiotherapy. However, the major differences between the TPSs for BNCT and the TPSs for conventional radiotherapies are as follows:
- The particle transport calculation for both neutrons and photons is generally carried out by using a Monte Carlo method. In recent years, the Monte Carlo method for dose calculation has also been applied to the TPSs for conventional radiotherapies such as X-ray therapy and proton radiotherapy, in order to obtain dose calculation results more accurately. However, the Monte Carlo method in the TPSs of conventional radiotherapy has been specialized to track the individual particle paths scattered in materials, and simplified to be able to perform dose estimation with a reasonably short time, applicable to routine treatment planning work. Hence, this method is called the “Simplified Monte Carlo method” (18,19). On the other hand, for the TPSs of BNCT, normal Monte Carlo based the dose calculation method has been generally employed, because the behavior of neutrons is diverse and complicated, and the dose caused by secondary radiation must also be considered. The normal Monte Carlo method is also called the “Full Monte Carlo method” in contradistinction to the Simplified Monte Carlo method. “Monte Carlo method” in this report implies the “Full Monte Carlo method”.
- The time required for the particle transport calculation may be longer for TPSs for BNCT than that for TPSs for conventional radiotherapies owing to the requirement of the Monte Carlo calculation.
- The dose arising from the reactions between neutrons and the boron compound accumulated in cells has to be estimated. The concentration of boron-10 in each organ and tissue is different, and its biological effect is also different for each tissue.
The current TPSs for BNCT are only forward planning systems. Therefore, in treatment planning, multiple calculations under various irradiation conditions have to be implemented. Doctors choose the most valid irradiation conditions among these multiple calculation conditions, and they are applied to the actual treatment. Though the basic principles of an inverse planning system for BNCT have been proposed, it has not yet been put to practical use.
To implement the series of treatment planning steps described above, the main essential functions required for a TPS for BNCT are as follows:
- A loading function for the CT and MRI medical images. The format of the images is generally the DICOM format;
- Contouring of the target regions and ROIs from each medical image;
- Construction of a 3D model of a patient from the medical images including the definitions of the target volumes and ROIs;
- Selection of the neutron beam conditions with the patient’s 3D model;
- Conversion from the 3D model to a calculation model. In the current system, a voxel model is employed in the calculation;
- A radiation transport calculation with the model. In BNCT dosimetry, transport calculations for neutrons and photons are required, and the particle transport for neutrons and photons is generally determined by a Monte Carlo method. For the application of an external general-purpose Monte Carlo code to the particle transport calculation, an interface function with the code is necessary. The format for the input data for the voxel calculation model must match the format of the code;
- Interpolation of the calculation results with the voxel model to each pixel of the original medical images;
- Analysis of the interpolated calculation results for the dose and visualization of the results, such as a DVH and the two-dimensional distributions in CT images.
TPS for reactor-based BNCT
To carry out a clinical study using reactor-based BNCT facilities around the world, some TPSs for BNCT have been developed. Table 4 lists the major TPSs that have been used in actual clinical trials of reactor-based BNCT. In the table, new TPSs that are being developed in Japan for accelerator-based BNCT devices are also listed.
Table 4
| TPS | Developers | Transport code | Facility |
|---|---|---|---|
| NCTPlan | Harvard-MIT-CNEA | MCNP | MITR (MIT), RA6 (CNEA) |
| SERA | INEEL/MSU | seraMC (dedicated) | KUR (KURRI), FiR-1 (VTT), etc. |
| JCDS | JAEA | MCNP/PHITS | JRR-4 (JAEA) |
| THORplan | Tsing Hua Univ. | MCNP | THOR (Tsing Hua Univ.) |
| SACRA Planning | Sumitomo Heavy Industry Co. | PHITS | For accelerator-based device |
| Tsukuba-Plan | Univ. of Tsukuba | PHITS | For accelerator-based device |
BNCT, boron neutron capture therapy; TPS, treatment planning system.
These TPSs are noncommercial and have been developed as support tools for BNCT by small teams with expertise in nuclear engineering at some research institutes and universities related to BNCT. The first generation of TPSs was developed in the USA in the 1980s to the 1990s. During this time, some BNCT facilities capable of generating an epithermal neutron beam were constructed. The realization of irradiation with an epithermal neutron beam has enabled the irradiation of a deeper target. In past BNCT with a thermal neutron beam, the dose administered by the irradiation field was estimated by measurement using detectors such as gold foils placed within the irradiation field of the patient (20). However, in BNCT using an epithermal neutron beam, it is impossible to measure the dose directly at a deeper region in the body because detectors cannot be placed in the body. Hence, a TPS capable of estimating the dose at all places in the irradiation field, including in the body, have become necessary.
As listed in Table 4, four TPSs have been developed for reactor-based BNCT and put into practical use. First, the Massachusetts Institute of Technology (MIT) developed “NCTPlan” (21) in the 1990s. The system was developed by Harvard and an MIT group. For the Monte Carlo transport calculation, the system employed MCNP (9). In the particle transport calculation, NCTPlan creates a voxel calculation model consisting of 1×1×1 cm3 voxel cells from a 3D model constructed with a patient’s CT images. NCTPlan has been applied to treatment planning work in the BNCT clinical trials performed at MITR. The BNCT group at CNEA in Argentina has also employed NCTPlan for clinical studies for BNCT at the RA6 reactor in CNEA (22).
SERA (16) was developed by a collaboration between INEEL and Montana State University in 1999. SERA has been employed at many BNCT facilities such as the Kyoto University Research Reactor Institute (KURRI) (23), Helsinki University Hospital in Finland (24), and Studsvik in Sweden (25). SERA is still utilized in clinical trials that are being performed at KURRI. Thus, the system has a track record of treatment planning for hundreds of clinical cases. The voxel cell size for the calculation model of SERA is selectable to any size, which is independent from the minute resolution of the original patient model formed based on CT images. Thus, users can set an appropriate size with various conditions for dose estimation, though the size of 1×1×1 cm3, which is the same as that of NCTPlan, has most commonly been applied to clinical use.
In Japan, the JAEA Computational Dosimetry System (JCDS) (26) was developed by the Japan Atomic Energy Agency (JAEA) in order to realize clinical trials of BNCT by means of a medical facility installed at research reactor No. 4 (JRR-4). A clinical trial involving treatment planning with the JCDS at JRR-4 began in 2001 (27), and it was used for the treatment planning of approximately 100 cases that were implemented until the reactor was shut down in 2011. The first version of the JCDS employed MCNP as the Monte Carlo dose calculation engine. The voxel size can be chosen from several sizes for 2×2×2 mm3 (8 mm3), 5×5×5 mm3 (125 mm3), and 1×1×1 cm3 (1 cm3) voxel cells. In clinical use, the calculation model consisting of the 8 mm3 voxel cells had been usually used due to obtain more accurate dose calculation results (28). Thus, the calculation accuracy with the JCDS was expected to be better than those of other systems. However, there was a possibility that the calculation time would be longer than those of other systems. After that, the JCDS was improved to perform the particle transport calculation with the PHITS code in addition to MCNP in the mid-2000s. During this improvement, the second version of the JCDS was able to perform a calculation with a very precise voxel model consisting of pixel-based voxel cells.
THORplan (29) was developed by the BNCT research group at National Tsing Hua University in Taiwan in order to perform clinical trials at the THOR reactor installed at the university. The system also applies MCNP as the Monte Carlo dose calculation engine. Further, the voxel cell size of the calculation model in the system is 5×5×5 mm3 (125 mm3). Clinical trials with THORplan for recurrent head-and-neck cancer at the THOR reactor have been performed since 2010.
The conventional TPSs described above were developed as research tools for reactor-based BNCT at that time. Moreover, the systems have basic and minimum functions that can perform treatment planning for BNCT. However, in Japan, the laws related to pharmaceuticals and medical devices were revised in 2014, and the regulations for TPS have also changed. In the new pharmaceutical affairs law, every system used in radiation therapy that is carried out with advanced medical care or health insurance has to obtain pharmaceutical approval. In Japan, many accelerator-based BNCT treatment devices are already being developed and are expected to apply for pharmaceutical approval. Thus, it is expected that BNCT will be carried out with advanced medical care or health insurance in the near future. Therefore, it is necessary to produce new TPSs that can respond to accelerator-based BNCT and obtain pharmaceutical approval. Given this background, the University of Tsukuba is developing a new multimodal Monte-Carlo-based TPS (development code: Tsukuba-Plan) (30) based on the fundamental technologies of the JCDS. The Tsukuba-Plan has employed PHITS as a Monte Carlo transport code, whose application enables the determination of several doses arising from reactions with neutrons and photons as well as protons and heavy ions. Thus, the Tsukuba-Plan allows computational dosimetry for BNCT, particle radiotherapy, and X-ray therapy. At present, the verification for the dose estimation of the system is being performed.
In addition, Sumitomo Heavy Industries, Ltd. is developing a new TPS named “SACRA Planning” (31) that is applicable to a cyclotron-based BNCT device manufactured by the company. The Monte Carlo transport code of the SACRA Planning has also applied PHITS. The user interface of the SACRA Planning is being developed in collaboration with RaySearch Laboratories (RaySearch), which is one of the most famous manufacturers of TPSs for X-ray therapy. Neutron Therapeutics, Inc. (NTI), a venture company for accelerator-based BNCT treatment devices, is also developing a new TPS for BNCT, in collaboration with RaySearch. The venture company of CICS, Inc. is also developing original TPSs in order to realize treatments with their devices.
Patient’s positioning and monitoring
In modern external radiation therapies, the setup precision, inter-treatment position reproducibility, and intra-treatment position maintenance of the patient are important factors affecting the treatment effect. In order to implement radiation therapies based on the dose evaluation of a TPS, it is necessary to perform dose evaluation with the highly accurate calculations of the TPS and to reproduce the body position of the patient as much as possible with a preliminarily acquired CT image. Furthermore, it is necessary to maintain the same body posture during beam irradiation. In radiation therapies excluding BNCT, the patient lies straight on the treatment bed—the same as that during CT imaging. That is, irradiation from any angle is possible with a rotational gantry, and the burden on the patient is limited. However, in the current status of BNCT, the neutron beam has been limited to irradiation from a fixed irradiation port. Therefore, it is necessary to perform the setup process while changing the patient’s body axis and posture with respect to the neutron beam. Moreover, in the case of BNCT, a setup with the affected part close to the beam port is necessary in order to shorten the irradiation time as much as possible. Further, from the point of view of avoiding radiation exposure to the outside of the irradiation target region of the patient since neutrons emitted from the beam port diverge widely, it is necessary to immobilize the patient as close as possible to the beam port. Since the surroundings of the beam port include a shielding wall to prevent the leakage of radiation such as neutrons and photons upstream from the beam, there is a possibility that the patient’s body and wall will contact each other depending on the patient’s posture. These difficulties are exclusive to the setup of a BNCT treatment compared to other radiotherapies.
Patient positioning for BNCT treatment
Patient positioning is an indispensable process to realize an individual-optimized dose distribution for the patient that is pre-evaluated by a TPS. Several papers have been published regarding patient setup techniques for BNCT.
Wielopolski et al. reported a patient positioning and immobilization technique with a detailed procedure for the BNCT of glioblastoma multiforme at the Brookhaven Medical Research Reactor (32). The features of their method are summarized as follows:
- Using a mockup room for simulating patient treatment positioning, immobilization, and placing the markings for the final BNCT treatment positioning;
- Using a portable stereotactic frame for providing critical reference points for determining the entry point of the central beam axis of the irradiation beam and the patient positioning coordinates calculated relative to these points;
- Using patient support devices such as a table and chair for assuring comfort during a BNCT treatment.
Palmer et al. introduced a detailed procedure of patient positioning for BNCT treatment at Harvard-MIT (33), which is summarized as follows:
- Using Vac-Lok cushions and a safety belt;
- Immobilizing the head with Aquaplast thermoplastic mesh;
- Using an individualized template made from foam-core poster board material to establish the correct alignment of the patient positioning relative to the beam axis;
Kumada et al. reported a patient setting procedure for BNCT treatments at the Japan Atomic Energy Agency (JAEA) (26,34) with the following characteristics:
- Using a patient setting simulator system equipped with a multidirectional laser pointing system;
- Using a TPS to supply the 3D position data of the patient’s head surface;
- Using a 3D digitizer to perform measurements in the irradiation room in order to confirm that the actual head position is fixed at ideal coordinates.
Kiger et al. reported a positioning technique using a 3D digitizer to determine the correct beam entry point for the head of the patient (35). They concluded that for the method that determines the beam entry point by combining a reference point marked on a patient with a 3D digitizer, patient positioning provides a significant improvement compared with a conventional method.
Auterinen et al. introduced patient positioning using another approach at the FiR-1 BNCT facility (36). That is, shoulder recesses were constructed around the beam aperture in order to make patient positioning near the irradiation port easier. They also evaluated the exposure doses to the body in the presence or absence of the shoulder recesses. They concluded that the increase in the exposure dose to the body calculated by the Monte Carlo code due to the shoulder recesses was acceptable.
Monitoring of patient movement during treatment
Almost all of a BNCT treatment has been treated in a single fraction. Therefore, the irradiation time per fraction is naturally longer than that of other radiotherapy modalities. Displacement of the patient may occur during beam irradiation during a BNCT treatment. There are several papers reporting the effects of the displacement of the patient’s position on the dose during BNCT.
Wielopolski et al. reported assessment results of the sensitivity to the displacement of the patient’s position regarding the doses for BNCT treatment (32). They simulated displacements in the patient’s position in the lateral direction of 0.5, 1.0, and 2.0 cm and evaluated the case where the distance between the head between the face of the beam collimator occurred similarly. Their simulation results showed large dose variations for a displacement in a direction away from the port compared to a displacement in the lateral direction.
Takada et al. simulated the effect of the displacement of the patient’s position of less than 1 cm on the dose using the JRR-4 neutron beam (37). Their assumed displacement of the patient’s position was set to 1.5–9.0 mm laterally and 1.0–9.0 mm in the beam axis direction (air gap). Displacements of the patient’s position of several millimeters can be assumed in the cases where position fixation is insufficient or the irradiation time is long. According to their simulation results, a dose deviation of about 2% was observed when the displacement in the lateral direction was 9 mm. On the other hand, in their simulation for the direction away from the beam port, a dose deviation of about 10% was observed for 9 mm.
In general, when a displacement of the patient’s position occurs, the influence on dose is critical for well-collimated beams. The neutron beams used for BNCT are not well-collimated as much as those for conventional radiotherapy such as X-ray therapy. Moreover, the radiated epithermal neutrons rapidly decelerate in the patient’s body and spread all directions as thermal neutrons. Therefore, neutrons are widely irradiated around the irradiation site. Nevertheless, from these two results, it can be considered that displacements of the patient’s position in a direction away from the beam port affect the dose of BNCT.
It is assumed that the influence of the displacement of the patient’s position on the dose also changes depending on the beam divergence of BNCT beam and the irradiation site. Ideally, the displacements of the patient’s position during a BNCT treatment should be monitored in real-time and immediately reflected in the dose distribution calculation. Although there are several difficulties associated with realizing this, it is considered to be an important process to achieve higher accuracy irradiation during a BNCT treatment.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hiroaki Kumada and Yi-Wei Chen) for the series “Boron Neutron Capture Therapy” published in Therapeutic Radiology and Oncology. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2018.10.12). The series “Boron Neutron Capture Therapy” was commissioned by the editorial office without any funding or sponsorship. HK serves as an unpaid Guest Edior of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tanaka H, Sakurai Y, Suzuki M, et al. Characteristics comparison between a cyclotron-based neutron source and KUR-HWNIF for boron neutron capture therapy. Nucl Instrum Methods Phys Res B 2009;267:1970-7. [Crossref]
- Savolainen S, Kortesniemi M, Timonen M, et al. Boron neutron capture therapy (BNCT) in Finland: Technological and physical prospects after 20 years of experiences. Physica Medica 2013;29:233-48. [Crossref] [PubMed]
- Elowitz EH, Bergland RM, Coderre J A, et al. Biodistribution of p-boronophenylalanine in patients with glioblastoma multiforme for use in boron neutron capture therapy. Neurosurgery 1998;42:463-8; discussion 468-9. [Crossref] [PubMed]
- Goorley JT, Kiger WS 3rd, Zamenhof RG. Reference dosimetry calculations for neutron capture therapy with comparison of analytical and voxel models. Medical Physics 2002;29:145-56. [Crossref] [PubMed]
- Kiger WS 3rd, Palmer MR, Riley KJ, et al. A pharmacokinetic model for the concentration of 10B in blood after boronophenylalanine-fructose administration in humans. Radiat Res 2001;155:611-8. [Crossref] [PubMed]
- Shibata Y, Matsumura A, Yamamoto T, et al. Prediction of boron concentrations in blood from patients on boron neutron capture therapy. Anticancer Res 2003;23:5231-5. [PubMed]
- Coderre JA, Chanana AD, Joel DD, et al. Biodistribution of boronophenylalanine in patients with glioblastoma multiforme: boron concentration correlates with tumor cellularity. Radiat Res 1998;149:163-70. [Crossref] [PubMed]
- Pillay AE, Peisach M. Some studies on nuclear methods for boron determination. Nucl Instrum Methods Phys Res B 1992;66:562-6. [Crossref]
- Sah RN, Brown PH. Boron determination—a review of analytical methods. Microchem J 1997;56:285-304. [Crossref]
- Linko S, Revitzer H, Zilliacus R, et al. Boron detection from blood samples by ICP-AES and ICP-MS during boron neutron capture therapy. Scand J Clin Lab Invest 2008;68:696-702. [Crossref] [PubMed]
- Coderre JA, Morris MG. The radiation biology of boron neutron capture therapy. Radiat Res 1999;151:1-18. [Crossref] [PubMed]
- Yamamoto T, Matsumura A, Yamamoto K, et al. Characterization of neutron beams for boron neutron capture therapy: In-air radiobiological dosimetry. Radiat Res 2003;160:70-6. [Crossref] [PubMed]
- Shibata K, Iwamoto O, Nakagawa T, et al. JENDL-4.0: A new library for nuclear science and engineering. J Nucl Sci Technol 2011;48:1-30. [Crossref]
- Briesmeister JF. MCNP - A general Monte Carlo N-particle transport code version 4C. LA-13709-M, 2000.
- Iwase H, Niita K, Nakamura T. Development of general-purpose particle and heavy ion transport Monte Carlo code. J Nucl Sci Technol 2002;39:1142-51. [Crossref]
- Nigg DW, Wemple CA, Wessel DE, et al. SERA -An advanced treatment planning system for neutron therapy and BNCT. Trans Am Nucl Soc 1999;80:66-8.
- Chadwick MB, Obložinský P, Herman M, et al. ENDF/B-VII.0: next generation evaluated nuclear data library for nuclear science and technology. Nucl Data Sheets 2006;107:2931-3060. [Crossref]
- Takayanagi T, Hirayama S, Fujitaka S, et al. A Simplified Monte Carlo algorithm considering large-scattering for fast and accurate calculation of proton dose. J Appl Clin Med Phys 2018;19:60-72. [Crossref] [PubMed]
- Mizutani S, Takada Y, Kohno R, et al. Application of dose kernel calculation using a simplified Monte Carlo method to treatment plan for scanned proton beams. J Appl Clin Med Phys 2016;17:315-27. [Crossref] [PubMed]
- Nakagawa Y, Hatanaka H. Boron neutron capture therapy - Clinical brain tumors studies. J Neurooncol 1997;33:105-15. [Crossref] [PubMed]
- Zamenhof R, Redmond E, Solares G, et al. Monte Carlo-based treatment planning for boron neutron capture therapy using custom designed models automatically generated from CT data. Int J Radiat Oncol Biol Phys 1996;35:383-97. [Crossref] [PubMed]
- Provenzano L, Farias RO, Longhino J M, et al. A prospective study to assess the performance of the improved Boron Neutron Capture Therapy Facility in Argentina. Appl Radiat Isot 2014;88:171-6. [Crossref] [PubMed]
- Kato I, Ono K, Sakurai Y, et al. Effectiveness of BNCT for recurrent head and neck malignancies. Appl Radiat Isot 2004;61:1069-73. [Crossref] [PubMed]
- Kankaanranta L, Seppala T, Koivunoro H, et al. Boron neutron capture therapy in the treatment of locally recurred head-and neck cancer: final analysis of a phase 1/2 trial. Int J Radiat Oncol Biol Phys 2012;82:e67-75. [Crossref] [PubMed]
- Capala J, Stenstam BH, Skold K, et al. Boron neutron capture therapy for glioblastoma multiforme: clinical studies in Sweden. J Neurooncol 2003;62:135-44. [Crossref] [PubMed]
- Kumada H, Yamamoto K, Matsumura A, et al. Verification of the computational dosimetry system in JAERI (JCDS) for boron neutron capture therapy. Phys Med Biol 2004;49:3353-65. [Crossref] [PubMed]
- Yamamoto T, Nakai K, Kageji T, et al. Boron neutron capture therapy for newly diagnosed glioblastoma Radiother Oncol 2009;91:80-4. [Crossref] [PubMed]
- Kumada H, Yamamoto K, Matsumura A, et al. Development of JCDS, a computational dosimetry system at JAEA for boron neutron capture therapy. J. Phys: Conf Ser 2007;74:021010 [Crossref]
- Lin TY, Liu YH. Development and verification of THORplan - A BNCT treatment planning system for THOR. Appl Radiat Isot 2011;69:1878-81. [Crossref] [PubMed]
- Kumada H, Takada K, Sakurai Y, et al. Development of a multimodal Monte Carlo based treatment planning system. Radiat Prot Dosimetry 2018;180:286-90. [Crossref] [PubMed]
- Mukawa T, Yamauchi T, Aoki Y, et al. Development of treatment planning system for in-hospital BNCT system. Abstract of the 17th International Congress on Neutron Capture Therapy 2016;212.
- Wielopolski L, Capala J, Pendzick NE, et al. Patient positioning in static beams for boron neutron capture therapy of malignant glioma. Radiat Med 2000;18:381-7. [PubMed]
- Palmer MR, Goorley JT, Kiger WS, et al. Treatment planning and dosimetry for the Harvard-MIT Phase I clinical trial of cranial neutron capture therapy. Int J Radiat Oncol Biol Phys 2002;53:1361-79. [Crossref] [PubMed]
- Kumada H, Matsumura A, Nakagawa Y. Development of the patient setting system for medical irradiation with research reactor. Trans At Energy Soc Japan 2002;1:59-68. [Crossref]
- Kiger WS 3rd, Albritton JR, Lu XQ, et al. Development and application of an unconstrained technique for patient positioning in fixed radiation beams. Appl Radiat Isot 2004;61:765-9. [Crossref] [PubMed]
- Auterinen I, Kotiluoto P, Hippeläinen E, et al. Design and construction of shoulder recesses into the beam aperture shields for improved patient positioning at the FiR 1 BNCT facility. Appl Radiat Isot 2004;61:799-803. [Crossref] [PubMed]
- Takada K, Kumada H, Liem PH, et al. Development of Monte Carlo based real-time treatment planning system with fast calculation algorithm for boron neutron capture therapy. Phys Med 2016;32:1846-51. [Crossref] [PubMed]
Cite this article as: Kumada H, Takada K. Treatment planning system and patient positioning for boron neutron capture therapy. Ther Radiol Oncol 2018;2:50.


