
Role of radiotherapy in heterotopic ossification: a case report and literature review
Introduction
Heterotopic ossification (HO), the process of abnormal bone formation in non-osseous tissue, was first described by Riedel and was later reported by Dejerine and Ceillier in 1918 during World War I (1). Although it can develop at any site including the elbows, shoulders, knees, or other soft tissues associated with joints, the hip is the most common site of involvement (2,3). HO may limit the range of motion of joints and lead to stiffness, although a few patients might be diagnosed based only on radiographic findings in the absence of major clinical symptoms (3,4). Brooker et al. developed a classification for the assessment of HO, which is the most commonly used system globally (Table 1) (2). No specific etiological contributors have been identified in patients with HO; however, previous studies suggest the potential role of previous ipsilateral or contralateral HO, ankylosing spondylitis, hypertrophic osteoarthritis, spinal cord injury, or hereditary diseases as possible risk factors associated with this condition (3,4).
Table 1
| Stage | Description |
|---|---|
| I | Bone islands within the soft tissue |
| II | Bone spurs from the pelvis or proximal end of the femur, with at least 1 cm between opposing bone surfaces |
| III | Bone spurs from the pelvis and/or proximal end of the femur, with less than 1 cm between opposing bone surfaces |
| IV | Apparent bone ankylosis of the hip |
The incidence of HO found by radiographic images after hip arthroscopy ranges from 4.7% to 44% (5,6). Karunakar et al. reported that 37 (57.8%) of 64 patients with displaced fractures of the acetabulum exposed through a Kocher-Langenbeck approach had HO at 12 weeks after operation (7). The incidence of HO after hip arthroplasty is reported even up to 92% for patients who have a previous history of HO formation on the opposite side (8). Clinically, the incidence of significant HO (Brooker classification; grade III or grade IV with symptoms) following total hip replacement varies from 3% to 7% (9). Although non-steroidal anti-inflammatory drugs (NSAIDs) can be used as prophylactic agents against HO (10,11), these medications are contraindicated in patients with peptic ulcer, renal failure, or other comorbidities. Radiotherapy (RT) is an alternative treatment modality aimed at reducing the postoperative risk of development of HO (12). HO is a rare condition; thus, no globally accepted guidelines have been established for its management, and the dose and timing of the RT schedule/regimen vary among different hospitals/centers. We report a patient with HO who underwent adjuvant RT after the surgical resection. We also present a review of the literature describing clinical outcomes in patients with HO who receive variable RT regimens (variable doses and fractionations).
Case presentation (Figures 1,2)
A 52-year-old woman presented to our clinic with a 1-year history of progressive stiffness of her left hip. She reported history of hypertension, diabetes mellitus, and peptic ulcer, which were all controlled with regular medication intake. She also reported history of an old cerebrovascular accident 7 years prior to presentation, with residual left hemiplegia. She underwent T11–12 vertebroplasty secondary to compression fractures 3 years prior to presentation. Because she was bed-ridden, she resided in a nursing home and received regular rehabilitation therapy. During passive stretching exercise, she was observed to have developed limited motion of left hip flexion over a year prior to presentation. Following an episode of hydrocephalus with a decline in cognitive function, she underwent the placement of a ventriculoperitoneal shunt and after recovery, she was brought to our hospital for further management.
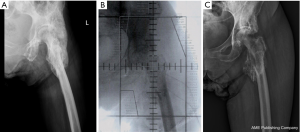
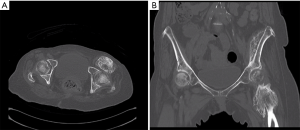
A pelvic X-ray showed a large amorphous soft tissue calcification at the left hip joint (Figure 1A). As shown in Figure 2, computed tomography (CT) revealed large ossified lesions at the left hip joint with compression of the adjacent muscular structures leading to the diagnosis of grade 4 HO. She underwent excision of the HO followed by RT because prophylactic use of NSAIDs was contraindicated in this patient owing to her underlying medical conditions.
The patient received a total dose of 10 Gray (Gy) delivered in 2 fractions over 2 consecutive days, within 48 hours of excision of the HO. We administered RT using conventional opposed anterior-posterior configurations to treat the left hip joint in this patient (Figure 1B). No acute adverse effects were reported after RT and following this treatment, she reported clinical improvement in her range of motion at the left hip, which improved up to 100° of motion. Figure 1C shows the post-irradiation X-rays demonstrating radiographic improvement in HO at the left hip. No recurrence of HO was identified at her 2-year follow-up after RT, and she was observed to be in a stable condition with a stationary range of motion of left hip flexion.
Discussion
Historically, HO was also termed ectopic ossification, para-osteoarthropathy, or myositis ossificans (13). Clinically, investigators have classified HO into three categories based on the risk factors associated with this condition: traumatic, neurogenic, and hereditary types (14). The traumatic type, or traumatic myositis ossificans, occurs after minor injuries, surgeries, or burns. This type usually develops in bones and joints in the vicinity of or surrounding the areas that are injured (14,15). The neurogenic type occurs as a result of neurological disorders, particularly in patients with severe spinal cord injuries (16). The hereditary type, or myositis ossificans progressiva, is the rarest variety attributable to a genetic condition, with an incidence of <1:1 million. The hereditary type of HO is associated with hereditary disorders such as fibrodysplasia ossificans progressiva, or Albright’s hereditary osteodystrophy; however, most patients succumb to restrictive lung disease or infection during their early life (13,17).
Although the etiology of HO is not fully understood, previous studies suggest that primitive mesenchymal cells get transformed into osteoblastic tissue leading to the process of lamellar bone formation in soft tissues (18). Surgical removal of the HO is recommended in those presenting with clinically apparent symptoms, and excision of the HO is aimed at maximizing the function of the affected joints (19). Prophylactic management against HO is needed to reduce further recurrence, although there is a lack of consensus regarding the optimal pharmacological management or prophylactic modality that is effective against HO (4,19).
Based on the action of NSAIDs, which inhibit uncontrolled activated cytokines that recruit osteoprogenitor cells, NSAIDs are considered effective chemoprophylactic agents against HO (10,11). Previous studies show that NSAIDs such as indomethacin or ibuprofen decrease the incidence of HO (20). However, limited data are available to show a significant benefit associated with the use of selective cyclo-oxygenase-2 inhibitors for the prevention of HO (21,22).
In addition to NSAIDs, RT may serve as useful alternative therapy for the prophylaxis of HO because RT inhibits proliferation of osteoprogenitor cells (12). Pakos et al. (23) performed a meta-analysis comprising seven randomized studies that compared between RT and NSAIDs as prophylactic modalities against HO and demonstrated that RT tended to be more effective than NSAIDs in preventing grade 3 or 4 HO (Brooker classification). Furthermore, Pakos et al. proposed that a dose of 6 Gy was as effective as NSAIDs in the prophylaxis of HO and that increasing the RT dose to >6 Gy was more effective than NSAIDs in the management of HO (23). These results suggest that RT is an effective alternative prophylactic modality for HO in patients with comorbidities in whom the use of NSAIDs is contraindicated.
Previous studies have described that the dose fractionation for RT for HO prophylaxis is variable (12,23-27). In the 1980s, a conventionally fractionated dose of 20 Gy was delivered based on extrapolation of bony growth inhibition according to application of RT in pediatric patients (12). Considering the potential adverse effects of RT, an increasing trend toward a reduced dose of RT for the prophylaxis of HO is being observed. Calculation of biologically effective dose (BED) values of RT for HO was based on the generic late effects α/β ratio of 3 (28). Sylvester et al. observed that the effects of HO prophylaxis and the adverse effects of RT were not significantly different between patients who received 10 Gy delivered in 5 fractions (BED =16.67 Gy) and those who received 20 Gy delivered in 10 fractions (BED =33.3 Gy) (24). Moreover, a single-fraction regimen comprising the administration of 7–8 Gy (BED =23.33–29.33 Gy) has been reported for the prophylaxis of HO (25,26). However, a low single-fraction dose of 5.5 Gy (BED =15.58 Gy) is not recommended for the prophylaxis of HO because of the higher radiographic failure rate (63%) than that observed with the administration of a single-fraction dose of 7 Gy (BED =23.33 Gy) (10%) (27). Table 2 summarizes the relevant series of radiation dose to HO prophylaxis after hip surgery (24-27).
Table 2
| Reference | Case number | Dose and fraction (n = number of irradiated hips) | Recurrence rate | Interpretation |
|---|---|---|---|---|
| Sylvester |
27 hips (26 patients) | 20 Gy in 10 fractions (n=8); |
25% (2/8) in 20 Gy group; |
10 Gy is effective compared to high dose of 20 Gy in conventional fractionation |
| Lo |
24 hips (23 patients) | 7 Gy in one fractions (n=24) | 4% (1/24) | Short-course radiotherapy results in good HO prophylaxis |
| Pellegrini |
62 hips (55 patients) | 8 Gy in 1 fractions (n=34); |
21% (7/34); 21% (6/28) | Single fractions with 8 Gy leads to comparative results of 10 Gy in conventional radiotherapy |
| Healy |
107 hips (94 patients) | 5.5 Gy in one fractions (n=19); |
63% (12/19); 10% (9/88) | Single fractions with 5.5 Gy was insufficient and results in high HO recurrence |
*, defined as Brooker grade 3 or above heterotopic ossification recurrence.
Previous studies have also described the use of preoperative RT for HO prophylaxis (28-31). Milakovic et al. (28) reviewed 12 randomized trials that described the use of RT for the prophylaxis of HO and demonstrated no significant difference between the administration of post or preoperative RT in preventing the progression of HO. Additionally, the BED (α/β =3) of ≤ 25 Gy in the efficacies of HO prophylaxis was not significant when compared with a BED >25 Gy (P=0.28) (28). RT inhibits the transformation of primitive mesenchymal cells into mature osteoblastic tissue. Thus, RT ought to be administered as early as possible during the clinical course of the patient after undergoing the trauma or operation (32,33). Mourad et al. retrospectively analyzed 585 patients with displaced acetabular fractures who underwent an operation followed by RT using a single-fraction 7 Gy dose (BED =23.33 Gy) for the prevention of HO and observed that 106 patients (18%) developed HO within the irradiated field (34). The authors showed that the risk of the failure rate increased from 10% for RT delivered within 3 days to 92% for RT delivered >21 days after the initial injury (34). Roos et al. also found that the incidences of HO for BED 9–18 Gy group, BED 20–24 Gy group, and BED 29–38 Gy were 23.4%, 14.8% and 15.8%, respectively (35). In this report, our patient received immediate RT within 48 hours after excision of HO with a total dose of 10 Gy in 2 fractions (BED =26.67 Gy). The patient demonstrated clinical improvement in her range of motion at the left hip without any recurrence of HO at 2-year follow-up. Taken together, these findings indicate that the postoperative timing of RT (BED at least ≥20 Gy) delivery may play an important role in determining the efficacy of RT for the prophylaxis of HO.
Conclusions
In summary, our case report indicates that the administration of 10 Gy delivered in 2 fractions within 2 days postoperatively is an effective alternative prophylactic against HO. Although HO is a rare disease, our results and those of previous studies demonstrate that the administration of RT using 10 Gy delivered in 2 fractions or 7 Gy delivered as a single fraction within 3 days postoperatively is a safe and effective treatment option for the prophylaxis of HO, particularly in patients in whom the use of medications is contraindicated for prophylaxis.
Acknowledgments
Funding: This study was supported by the following research grants: 107-S3797 and 107-S3883 from National Taiwan University Hospital.
Footnote
Conflicts of Interest: SHK serves as an Associate Editor-in-Chief of Therapeutic Radiology and Oncology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dejerine A, Ceillier A. Para-osteo-arthropathies des paraplegigues par lesion medullarie; etude clinique et radiographique. Ann Med 1918;5:497.
- Brooker AF, Bowerman JW, Robinson RA, et al. Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg Am 1973;55:1629-32. [Crossref] [PubMed]
- Ranganathan K, Loder S, Agarwal S, et al. Heterotopic Ossification: Basic-Science Principles and Clinical Correlates. J Bone Joint Surg Am 2015;97:1101-11. [Crossref] [PubMed]
- Edwards DS, Kuhn KM, Potter BK, et al. Heterotopic Ossification: A Review of Current Understanding, Treatment, and Future. J Orthop Trauma 2016;30:S27-30. [Crossref] [PubMed]
- Bedi A, Zbeda RM, Bueno VF, et al. The incidence of heterotopic ossification after hip arthroscopy. Am J Sports Med 2012;40:854-63. [Crossref] [PubMed]
- Rath E, Sherman H, Sampson TG, et al. The incidence of heterotopic ossification in hip arthroscopy. Arthroscopy 2013;29:427-33. [Crossref] [PubMed]
- Karunakar MA, Sen A, Bosse MJ, et al. Indometacin as prophylaxis for heterotopic ossification after the operative treatment of fractures of the acetabulum. J Bone Joint Surg Br 2006;88:1613-7. [Crossref] [PubMed]
- DeLee J, Ferrari A, Charnley J. Ectopic bone formation following low friction arthroplasty of the hip. Clin Orthop Relat Res. 1976;53-9. [PubMed]
- Board TN, Karva A, Board RE, et al. The prophylaxis and treatment of heterotopic ossification following lower limb arthroplasty. J Bone Joint Surg Br 2007;89:434-40. [Crossref] [PubMed]
- Macfarlane RJ, Ng BH, Gamie Z, et al. Pharmacological treatment of heterotopic ossification following hip and acetabular surgery. Expert Opin Pharmacother 2008;9:767-86. [Crossref] [PubMed]
- Yeung M, Jamshidi S, Horner N, et al. Efficacy of Nonsteroidal Anti-inflammatory Drug Prophylaxis for Heterotrophic Ossification in Hip Arthroscopy: A Systematic Review. Arthroscopy 2016;32:519-25. [Crossref] [PubMed]
- Balboni TA, Gobezie R, Mamon HJ. Heterotopic ossification: Pathophysiology, clinical features, and the role of radiotherapy for prophylaxis. Int J Radiat Oncol Biol Phys 2006;65:1289-99. [Crossref] [PubMed]
- Iorio R, Healy WL. Heterotopic ossification after hip and knee arthroplasty: risk factors, prevention, and treatment. J Am Acad Orthop Surg 2002;10:409-16. [Crossref] [PubMed]
- Nauth A, Giles E, Potter BK, et al. Heterotopic ossification in orthopaedic trauma. J Orthop Trauma 2012;26:684-8. [Crossref] [PubMed]
- Punzi L, Galozzi P, Luisetto R, et al. Post-traumatic arthritis: overview on pathogenic mechanisms and role of inflammation. RMD Open 2016;2:e000279 [Crossref] [PubMed]
- Sullivan MP, Torres SJ, Mehta S, et al. Heterotopic ossification after central nervous system trauma: A current review. Bone Joint Res 2013;2:51-7. [Crossref] [PubMed]
- Ahrengart L, Lindgren U. Heterotopic bone after hip arthroplasty. Defining the patient at risk. Clin Orthop Relat Res 1993;153-9. [PubMed]
- Reddi AH. Bone morphogenetic proteins, bone marrow stromal cells, and mesenchymal stem cells. Maureen Owen revisited. Clin Orthop Relat Res 1995;115-9. [PubMed]
- Hoyt BW, Pavey GJ, Potter BK, et al. Heterotopic ossification and lessons learned from fifteen years at war: A review of therapy, novel research, and future directions for military and civilian orthopaedic trauma. Bone 2018;109:3-11. [Crossref] [PubMed]
- Fransen M. Group HMCotHC. Preventing chronic ectopic bone-related pain and disability after hip replacement surgery with perioperative ibuprofen. A multicenter, randomized, double-blind, placebo-controlled trial (HIPAID). Control Clin Trials 2004;25:223-33. [Crossref] [PubMed]
- Barthel T, Baumann B, Noth U, et al. Prophylaxis of heterotopic ossification after total hip arthroplasty: a prospective randomized study comparing indomethacin and meloxicam. Acta Orthop Scand 2002;73:611-4. [PubMed]
- Saudan M, Saudan P, Perneger T, et al. Celecoxib versus ibuprofen in the prevention of heterotopic ossification following total hip replacement: a prospective randomised trial. J Bone Joint Surg Br 2007;89:155-9. [Crossref] [PubMed]
- Pakos EE, Ioannidis JP. Radiotherapy vs. nonsteroidal anti-inflammatory drugs for the prevention of heterotopic ossification after major hip procedures: a meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys 2004;60:888-95. [Crossref] [PubMed]
- Sylvester JE, Greenberg P, Selch MT, et al. The use of postoperative irradiation for the prevention of heterotopic bone formation after total hip replacement. Int J Radiat Oncol Biol Phys 1988;14:471-6. [Crossref] [PubMed]
- Lo TC, Healy WL, Covall DJ, et al. Heterotopic bone formation after hip surgery: prevention with single-dose postoperative hip irradiation. Radiology 1988;168:851-4. [Crossref] [PubMed]
- Pellegrini VD Jr, Konski AA, Gastel JA, et al. Prevention of heterotopic ossification with irradiation after total hip arthroplasty. Radiation therapy with a single dose of eight hundred centigray administered to a limited field. J Bone Joint Surg Am 1992;74:186-200. [Crossref] [PubMed]
- Healy WL, Lo TC, DeSimone AA, et al. Single-dose irradiation for the prevention of heterotopic ossification after total hip arthroplasty. A comparison of doses of five hundred and fifty and seven hundred centigray. J Bone Joint Surg Am 1995;77:590-5. [Crossref] [PubMed]
- Milakovic M, Popovic M, Raman S, et al. Radiotherapy for the prophylaxis of heterotopic ossification: A systematic review and meta-analysis of randomized controlled trials. Radiother Oncol 2015;116:4-9. [Crossref] [PubMed]
- Kolbl O, Knelles D, Barthel T, et al. Preoperative irradiation versus the use of nonsteroidal anti-inflammatory drugs for prevention of heterotopic ossification following total hip replacement: the results of a randomized trial. Int J Radiat Oncol Biol Phys 1998;42:397-401. [Crossref] [PubMed]
- van Leeuwen WM, Deckers P, de Lange WJ. Preoperative irradiation for prophylaxis of ectopic ossification after hip arthroplasty. A randomized study in 62 hips. Acta Orthop Scand 1998;69:116-8. [Crossref] [PubMed]
- Seegenschmiedt MH, Keilholz L, Martus P, et al. Prevention of heterotopic ossification about the hip: final results of two randomized trials in 410 patients using either preoperative or postoperative radiation therapy. Int J Radiat Oncol Biol Phys 1997;39:161-71. [Crossref] [PubMed]
- Craven PL, Urist MR. Osteogenesis by radioisotope labelled cell populations in implants of bone matrix under the influence of ionizing radiation. Clin Orthop Relat Res 1971;231-43. [Crossref] [PubMed]
- Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med 2002;43:346-53. [PubMed]
- Mourad WF, Packianathan S, Shourbaji RA, et al. A prolonged time interval between trauma and prophylactic radiation therapy significantly increases the risk of heterotopic ossification. Int J Radiat Oncol Biol Phys 2012;82:e339-44. [Crossref] [PubMed]
- Roos DE, Smith JG. Radiotherapy for the prophylaxis of heterotopic ossification: A single 7-8 Gy fraction seems optimal. Radiother Oncol 2015;116:1-3. [Crossref] [PubMed]
Cite this article as: Huang MS, Chen YH, Kuo SH. Role of radiotherapy in heterotopic ossification: a case report and literature review. Ther Radiol Oncol 2018;2:41.


