Prospects for the new era of boron neutron capture therapy and subjects for the future
Brief history of boron neutron capture therapy (BNCT) research
Eighty two years has passed since the idea of BNCT was devised in 1936 and in 1951 the clinical research started in USA (1,2). The cytocidal effect of α particle and lithium nucleus released by the reaction of 10B(n,α)7Li is confined to the cells where the reaction occurred and relative biologic effectiveness (RBE) is very large, so it can also be applied to cancers to which conventional radiotherapy is less effective. As many clinical studies showing such an excellent effect are reported, it finally came to attract the public's attention (3-13).
However, unlike other particle beam therapies or X-ray therapy, 4 kinds of radiation are involved, and so, the mechanism of BNCT is complicated. Except gamma rays, particles through the reactions of 10B(n,α)7Li, 14N(n,p)14C and 1H(n,n’) 1H are high linear energy transfer (LET) radiations with high RBE. We must decide the RBE of each such radiation. Since nitrogen and hydrogen are homogeneously distributed in both tumor and normal tissue in general, it is easy to find it. On the other hand, the microscopic distribution of the 10B compound is not always homogeneous. 10B concentration in a tissue is an averaged macroscopic value. Then effective RBE of 10B(n,α)7Li reaction depends on the state of microscopic distribution of 10B drug. Therefore, this effective RBE, named compound biological effectiveness (CBE), varies depending on the kinds of boron compound, the target tissue and end points (14). Due to its nature, there is no way other than experiments to determine them.
For successful BNCT, boron compound is an absolutely important element. Only BPA is in full use clinically, and historically BSH was used but its role is now considered limited. Although some have been newly developed and their usefulness was experimentally validated, we do not know whether they will progress to full-scale clinical use. Under such circumstances, the development of an accelerator neutron source is steadily pioneering the new era of BNCT. In this paper, I will introduce the current state of Japan which is the most advanced in the world and prospect the future of BNCT.
Development of accelerator BNCT system
Worldwide, malignant brain tumor and head and neck cancer are the most common clinical cases of BNCT, and BNCT effectiveness is suggested in both cancers. The number of BNCT cases in the Kyoto University research reactor (KUR) is remarkably large among the research reactors in Japan. Since the all treated tumors already exceed 550 cases, we negotiated to make the reactor BNCT a treatment approved by the Ministry of Health and Welfare. However, it turned out that there is no possibility the research reactor to be approved as a medical device in Japan. Also, as long as we use research reactor for BNCT, there is limit in terms of sites where we can install it. This point becomes a major obstacle to make BNCT common treatment. The circumstances surrounding the research reactor seems to be similar in any place in the world. Therefore, the BNCT research group of Kyoto University officially decided to develop accelerator neutron irradiation system specialized for BNCT in 2007.
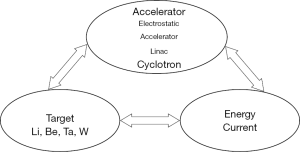
Accelerated protons are collided with various target metals for the generation of neutron in general. Accelerator, target metal, proton energy and current are mutually related elements (Figure 1). Various projects of accelerator BNCT system are in progress or under planning in Japan (Table 1). However, only the system jointly developed by the BNCT research group of Kyoto University Reactor Research Institute (re-renamed as the Institute for Integrated Radiation and Nuclear Science on April 1st: KURNS) and Sumitomo Heavy Industries, Ltd. has advanced to clinical test for cancer patients. The entry of the patients for second phase clinical test of recurrent malignant brain tumor and H&N cancers have completed and it is in the observation period. In view of these circumstances, I will introduce this system in comparison with other plans below.
Table 1
| Phase of development | Research Inst. or Hospital | Kind of Accelerator | Energy: MeV (current: mA) | Target |
|---|---|---|---|---|
| Under clinical test | KURNS (KURRI) | Cyclotron | 30 (~2) | Be |
| Under clinical test | Southern Tohoku BNCT Research Center | Cyclotron | 30 (~2) | Be |
| Waiting clinical test | Kansai BNCT Medical Center | Cyclotron | 30 (~2) | Be |
| Waiting clinical test | National Cancer Center Hospital | Linear accelerator | 2.5 [12] | 7Li(Solid) |
| Waiting clinical test | Tsukuba Uni./KEK | Linear accelerator | 8 (1.4) | Be |
| Under construction | Edogawa Hospital BNCT Center | Linear accelerator | 2.5 [12] | 7Li(Solid) |
| Under development | Nagoya University | Electro-static accelerator | 2.8 [15] | 7Li(Solid) |
BNCT, boron neutron capture therapy.
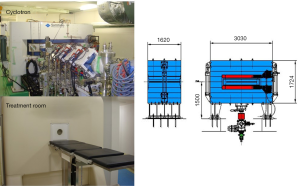
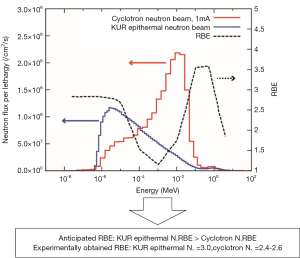
The accelerator is a cyclotron. Proton energy and current are 30 MeV, ~2 mA, respectively. The target is beryllium. There are no problems on size of the system, including the systems for degradation of neutron energy and irradiation, for setting up in clinical hospital (Figure 2) (15,16). Compared with the accelerator for proton beam therapy, although the acceleration energy is low, the current is several ten thousand or more times larger, so the amount of heat generated in the target is enormous. It is safe to use metals with high melting point and high thermal conductivity. Furthermore, depending on the proton energy, the protons stop in the metal target, accumulate as hydrogen gas and rapidly weaken and destroy the target (blistering). So, in order to solve both problems, we chose the metal with high melting point for the target and high energy proton with high efficiency of neutron generation. To solve both problems at the same time, we selected beryllium for the target metal and 30 MeV proton beam with a high efficiency of neutron generation. Since the range of 30 MeV protons in beryllium is 5.8 mm, we set the target thickness at 5.5 mm. 5.5 mm thick beryllium has sufficient strength and it is not necessary to reinforce the target from the back side with supporting metal. Then, it is also possible to directly cool the target. Since protons penetrate the beryllium target and fall into the cooling water, blistering can be easily and completely avoided. In the case of using a low energy proton beam, the beryllium target needs to be thin in order to avoid blistering. Inevitably, support metal that reinforces the strength is required. Furthermore, to avoid blistering of the supporting metal, it is also necessary to join the hydrogen absorbing metal. Naturally, the life of the target will also have to be short. In our system, the thermal neutron fluence rate obtained by 1 mA operation was 1.22×109 ncm−2s−1, which was 1.88 times larger than that of KUR, and the energy spectrum shifted to the high energy side (Figure 3).
This energy shift contributes to improvement of deep thermal neutron distribution while there is a negative point that the surface dose increases. The thermal neutron fluence rate in the deep part is twice as high as that obtained in heavy water facility of KUR. Depending on the shift of energy spectrum, of course, changes in RBE were anticipated. When examined using several cell lines, the RBE was 2.2 to 2.6, which was smaller than the value 3.0 obtained for the KUR epithermal neutron beam. Because the neutron energy became high, the proton energy released by the collision with the hydrogen nucleus also increased. Conversely, the LET of these protons became low and the RBE was also small. This was as anticipated (Figure 3).
We started Phase I clinical test of BNCT for recurrent malignant glioma from 2012 after experiment studies to confirm the effect of combined use with BPA, followed by recurrent or locally advanced inoperable head and neck cancers. At the moment writing this paper, it is in the observation period after phase II clinical test for both tumors. So, it will take more time to apply BNCT as an approved treatment to the Ministry of Health, Labour and Welfare in Japan.
Research subjects for future BNCT development
I describe the research subjects under the forecast of the near future that the boron drug BPA will continue to be the main drug in clinical BNCT.
Highly accurate prediction of dynamics of BPA in tumor and normal tissue
The degree of selectivity of accumulation of boron drugs in tumor is a very important factor. BPA can be labeled with a positron emitting nuclide 18F and its accumulation can be imaged and quantified with PET (17,18). If 18FBPA PET can be used as a pre-BNCT test, indication of BNCT can be decided based on PET data, so it may be unnecessary to conduct a clinical test for each type of cancer. This is the reason why approval of 18FBPA PET is essential for the development of BNCT. However, from the viewpoint of accuracy required for radiotherapy, it is necessary to further improve the accuracy of prediction of boron concentration and accumulation ratio with 18FBPA PET. It is considered that the change with time of boron concentration after administration of BPA varies depending on the tumor and normal tissues (19,20). Dynamic PET Study is indispensable for obtaining these information (21). Currently, in many clinical research and test, administration of BPA is continued even during neutron irradiation in order to keep the blood 10B concentration constant. However, the timing of neutron irradiation to maximize the selective antitumor effect may be different for each patient’s tumor. Such information should be taken for each tumor before BNCT. In addition, it is expected that the allowable duration of time to maximize the selective effect may be short. In order to cope with this restriction, the neutron fluence rate (ncm−2s−1) has to be high, which also leads to the subject for increasing the neutron intensity performance of the accelerator.
Furthermore, in human tumors, unlike experimental tumors, interstitial components are generally rich, and their amount varies considerably depending on the tumor. Concentration that can be measured with PET is just averaged macroscopic BPA level and BPA does not accumulate in interstitium, so in tumors with much interstitial tissues, there is a possibility of underestimating the BPA in tumor cells and overlooking the indication case. It is necessary to solve this point and achieve highly accurate prediction method of BPA concentration in tumor.
Enhancement of neutron fluence rate
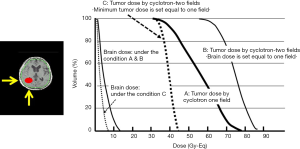
It is required to increase the neutron fluence rate capable of irradiation. Treatment will change drastically if it increases to about twice the present. We are operating the accelerator at 1 mA, but its performance is confirmed as being able to operate at 2 mA. There is also actual result of generating neutrons by operating at 1.5 mA. In the case of malignant glioma, irradiation time is shortened to half, about 20 minutes when operated at 2 mA. It is also possible to irradiate consecutively from two directions with single administration of BPA. By this, it is possible to raise the lowest dose of tumor dramatically or drastically lower the dose of normal brain by the irradiation from two directions (Figure 4).
Determining the CBE factor of BPA for normal tissues
In order to quantitatively evaluate the effect on normal tissues in BNCT, it is essential to know the CBE factor for each boron drug and normal tissue. However, even in BPA and BSH already in use, many CBE factors are yet to be confirmed (Table 2) (22). It is not possible to target cancers of organs or tissues for which the CBE factor has not been determined. For muscle, bone, intestine, kidney and liver, the CBE factor is uncertain. While there are organs that are mainly subject to the development of acute disorders that are relatively easy to study, there are also organs where chronic disorders are mainly expressed, so considerable difficulties are expected in the research.
Table 2
| Radiation | Tumor | Brain | Skin | Mucosa | Lung | Liver |
|---|---|---|---|---|---|---|
| Thermal N. | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Epithermal N. | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| 10B(n,α)7Li | ||||||
| BPA | 3.8 | 0.32+ N/B ×1.65 | 2.5 (0.73) | 4.9 | 0.32+ N/B ×1.80 | 4.3 (ND) |
| BSH | 2.5 | 0.36 | 0.8 (0.86) | 0.3 | ND | 0.9 (ND) |
|
|
1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
(····): CBE factors for late response. The formulas were published in J Radiat Res 2016;57 Suppl 1:i 83-i89. RBE, relative biologic effectiveness; CBE, compound biological effectiveness; BPA, paraboronophenylalanine; BSH, undecahydrododecaborate disodium; ND, not determined.
Development of new boron drugs
Unlike the above three subjects, this is a considerably long-lasting task. The goal is to develop a new boron drug that shows a higher tumor: normal tissue (blood) ratio than current boron drugs (BSH, BPA) and accumulates in the tumor at high concentration. In addition, the important requirement for boron drugs is the property of being uniformly distributed in the tumor. Because the range of emitted particles is extremely short, nonuniform distribution induces extreme inhomogeneities in the dose and causes tumor recurrence. Boron chemists and pharmacists have a very important role and responsibility for the future development of BNCT. Although various boron compounds have been proposed, many of them except some are still in the basic accumulation test stage, and strict microscopic distribution tests or detailed verification of the effect of neutron irradiation combined is not performed (23-29). Furthermore, in many of these compounds, it has not been studied how much influence occurs in normal tissues when combined with neutron irradiation.
Conclusions for future prospects
Radiotherapy of cancers and their techniques by X-rays and particle beams (proton beams, heavy ion beams) are now generally almost established. The current reaching point can be summarized as follow. Firstly, it was found that cancers localized to some extent can be completely controlled by focused radiation beam, and adverse events can also be avoided. Secondly, the definite efficacy of high LET radiation against X-ray resistant tumors was demonstrated in heavy particle radiation therapy. Third, technology to track and irradiate cancers of organs with physiological movement has been developed. On the other hand, BNCT has all of the above three reaching points with high accuracy, as can be understood from its principle. The development of superior boron drugs in the future should further improve these. In that sense, BNCT has no limit to its development.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Locher GL. Biological effects and therapeutic possibilities of neutron. AJR 1936;36:1-13.
- Sweet WH, Javid M. The possible use of neutron-capturing isotopes such as boron-10 in the treatment of neoplasms. I. Intracranial tumor. J Neurosurg 1952;9:200-9. [Crossref] [PubMed]
- Hatanaka H, Kamano S, Amano K, et al. Clinical experience of boron-neutron capture therapy for gliomas - a comparison with conventional chemo-immure-radiotherapy. In: Hatanaka H. editor. Boron-neutron capture therapy for tumors. Niigata: Nishimura, 1986:349-53.
- Mishima Y, Honda C, Ichihashi M, et al. Treatment of malignant melanoma by single thermal neutron capture therapy with melanoma-seeking 10B-compound. Lancet 1989;2:388-9. [Crossref] [PubMed]
- Menéndez PR, Roth BM, Pereira MD, et al. BNCT for skin melanoma in extremities: updated Argentine clinical results. Appl Radiat Isot 2009;67:S50-3. [Crossref] [PubMed]
- Miyatake S, Tamura Y, Kawabata S, et al. Boron neutron capture therapy for malignant tumors related to meningiomas. Neurosurgery 2007;61:82-90. [Crossref] [PubMed]
- Miyatake S, Kawabata S, Yokoyama K, et al. Survival benefit of boron neutron capture therapy for recurrent malignant gliomas. J Neurooncol 2009;91:199-206. [Crossref] [PubMed]
- Kawabata S, Miyatake S, Nonoguchi N, et al. Survival benefit from boron neutron capture therapy for the newly diagnosed glioblastoma patients. Appl Radiat Isot 2009;67:S15-8. [Crossref] [PubMed]
- Kato I, Ono K, Sakurai Y, et al. Effectiveness of BNCT for recurrent head and neck malignancies. Appl Radiat Isot 2004;61:1069-73. [Crossref] [PubMed]
- Kankaanranta L, Seppälä T, Koivunoro H, et al. Boron neutron capture therapy in the treatment of locally recurred head-and-neck cancer: final analysis of a phase I/II trial. Int J Radiat Oncol Biol Phys 2012;82:e67-e75. [Crossref] [PubMed]
- Wang LW, Wang SJ, Chu PY, et al. BNCT for locally recurrent head and neck cancer: preliminary clinical experience from a phase I/II trial at Tsing Hua Open-Pool Reactor. Appl Radiat Isot 2011;69:1803-6. [Crossref] [PubMed]
- Suzuki M, Endo K, Satoh H, et al. A novel concept of treatment of diffuse or multiple pleural tumors by boron neutron capture therapy (BNCT). Radiother Oncol 2008;88:192-5. [Crossref] [PubMed]
- Suzuki M, Sakurai Y, Hagiwara S, et al. First attempt of boron neutron capture therapy (BNCT) for hepatocellular carcinoma. Jpn J Clin Oncol 2007;37:376-81. [Crossref] [PubMed]
- Morris GM, Coderre JA, Hopewell JW, et al. Response of rat skin to boron neutron capture therapy with p-boronophenylalanine or borocaptate sodium. Radiother Oncol 1994;32:144-53. [Crossref] [PubMed]
- Tanaka H, Sakurai Y, Suzuki M, et al. Improvement of dose distribution in Phantom by using epithermal neutron source based on the Be (p, n) reaction using a 30 MeV proton cyclotron accelerator. Appl Radiat Isot 2009;67:S258-61. [Crossref] [PubMed]
- Tanaka H, Sakurai Y, Suzuki M, et al. Experimental verification of beam characteristics for cyclotron-based epithermal neutron source (C-BENS). Appl Radiat Isot 2011;69:1642-5. [Crossref] [PubMed]
- Imahori Y, Ueda S, Ohmori Y, et al. Advances in Brief Positron emission tomography based boron neutron capture therapy using boronophenylalanine for high grade gliomas: Part-I. Clin Cancer Res 1998;4:1825-32. [PubMed]
- Hanaoka K, Watabe T, Naka S, et al. FBPA PET in boron neutron capture therapy for cancer: prediction of 10B concentration in the tumor and normal tissue in a rat xenograft model. EJNMMI Res 2014;4:70. [Crossref] [PubMed]
- Fukuda H, Honda C, Wadabayashi N, et al. Pharmacokinetics of 10 B - p - boronophenylalanine in tumours, skin and blood of melanoma patients: a study of boron neutron capture therapy for malignant melanoma. Melanoma Res 1999;9:75-83. [Crossref] [PubMed]
- Imahori Y, Ueda S, Ohmori Y, et al. Advances in Brief Positron emission tomography based boron neutron capture therapy using boronophenylalanine for high grade gliomas: Part-II. Clin Cancer Res 1998;4:1833-41. [PubMed]
- Morita T, Kurihara H, Hiroi K, et al. Dynamic changes in 18F-borono-L-phenylalanine uptake in unresectable, advanced, or recurrent squamous cell carcinoma of the head and neck and malignant melanoma during boron neutron capture therapy patient selection. Radiat Oncol 2018;13:4. [Crossref] [PubMed]
- Ono K. An analysis of the structure of the compound biological effectiveness factor. J Radiat Res 2016;57 Suppl 1:i 83-i89.
- Trivillin VA, Heber EM, Itoiz ME, et al. Radiobiology of BNCT mediated by GB-10 and GB-10+BPA in experimental oral cancer. Appl Radiat Isot 2004;61:939-45. [Crossref] [PubMed]
- Koganei H, Ueno M, Tachikawa S, et al. Development of high boron content liposomes and their promising antitumor effect for neutron capture therapy of cancers. Bioconjug Chem 2013;24:124-32. [Crossref] [PubMed]
- Michiue H, Sakurai Y, Kondo N, et al. The acceleration of boron neutron capture therapy using multi-linked mercaptoundecahydrododecaborate (BSH) fused cell-penetrating peptide. Biomaterials 2014;35:3396-405. [Crossref] [PubMed]
- Hattori Y, Kusaka S, Mukumoto M, et al. Synthesis and in vitro evaluation of thiododecaborated α, α-cycloalkylamino acids for the treatment of malignant brain tumors by boron neutron capture therapy. Amino Acids 2014;46:2715-20. [Crossref] [PubMed]
- Nakamura H, Koganei H, Miyoshi T, et al. Antitumor effect of boron nitride nanotubes in combination with thermal neutron irradiation on BNCT. Bioorg Med Chem Lett 2015;25:172-4. [Crossref] [PubMed]
- Yanagie H, Dewi N, Higashi S, et al. Selective boron delivery by intra-arterial injection of BSH-WOW emulsion in hepatic cancer model for neutron capture therapy. Br J Radiol 2017;90:20170004 [Crossref] [PubMed]
- Futamura G, Kawabata S, Nonoguchi N, et al. Evaluation of a novel sodium borocaptate-containing unnatural amino acid as a boron delivery agent for neutron capture therapy of the F98 rat glioma. Radiat Oncol 2017;12:26. [Crossref] [PubMed]
Cite this article as: Ono K. Prospects for the new era of boron neutron capture therapy and subjects for the future. Ther Radiol Oncol 2018;2:40.