Effectiveness and acute toxicity of intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy in pancreatic cancer: a systematic review and a meta-analysis
Introduction
Pancreatic cancer is one of the most aggressive malignancies with a very poor prognosis. When first detected, about 40% of patients have distant metastases and 30–40% have locally advanced disease precluding resection (1). Only a relatively small fraction of patients (<20%) can be treated with resection (1,2). Chemoradiotherapy is the current standard treatment modality for patients with locally advanced pancreatic cancer, with some patients achieving secondary resectability after chemoradiotherapy (2).
Nevertheless, chemoradiotherapy of pancreatic cancer often causes toxicity that can lead to considerable loss of quality of life. The retroperitoneal position of the pancreas places the stomach and duodenum within the radiation field, and concurrent chemoradiation therapy (CCRT)-related toxicity of both organs become major dose-limiting factors (3,4). Acute gastrointestinal (GI) toxicity related to CCRT of pancreatic cancer includes nausea, vomiting, diarrhea, abdominal pain, and bleeding. Acute hematologic (HEMA) toxicity includes neutropenia, thrombocytopenia, or pancytopenia. Physicians usually evaluate GI and HEMA toxicity by credible criteria, as National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE). The toxicity tends to lower the patient’s quality of life, can delay the treatment of pancreatic cancer, and can be life-threatening (3,5).
In recent years, intensity-modulated radiotherapy (IMRT) has been proposed as an alternative to conventional 3D-conformal radiation therapy (3D-CRT). The rationale was a reduction of therapy-related toxicity due to improved sparing of normal tissue (4-6). However, several shortcomings of IMRT, such as higher cost, higher requirement for immobilization during treatment, and a longer period of planning, cannot be ignored (7). Many studies have reported the treatment outcomes of IMRT, with inconsistent results. Many of these studies were small in size, varied in patient staging, and lacked parallel comparison groups (8-14). Evidence on whether IMRT offers advantages over 3D-CRT remains inconclusive.
The aim of this study is thus to summarize the current evidence regarding the clinical effectiveness and safety of IMRT as compared with 3D-CRT. We conducted a systematic review and a meta-analysis with the primary research question of whether OS can be improved using IMRT as compared with 3D-CRT. The secondary aim was to derive comparative effect estimates on the organ toxicity, specifically GI and HEMA toxicity, in patients with pancreatic cancer.
Methods
Search strategy
We conducted a systematic literature search of intensity-modulated radiotherapy and pancreatic cancer using Medline (via PubMed) and EMBASE from 1975 through December 2017. Two authors (WT Hsu and CN Chang) independently performed the literature search. Medical Subject Headings (MeSH) and the EMBASE TREE tool (EMTREE) were used to guide the choice of appropriate search terms in other databases. The first query was made using the following exploded headings and independent terms: “intensity-modulated radiotherapy” OR “intensity-modulated radiation therapy” OR “intensity-modified radiotherapy”. The second query was made using exploded headings and independent terms for pancreatic cancer: “pancreatic cancer” OR “pancreatic neoplasm”. We restricted our search to adults (age >18 years). A similar search strategy and search terms were used in EMBASE. We did not set limitations on language, country, or publication date. To ensure a comprehensive literature review, manual searches were also performed using the reference lists of retrieved articles, conference abstracts, and other databases, including the Web of Science and Cochrane databases.
Inclusion and exclusion criteria
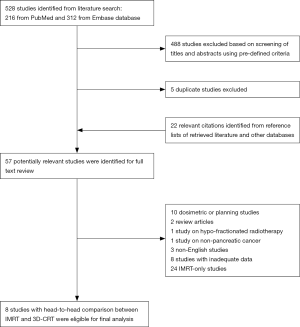
Study selection is summarized in Figure 1. Two reviewers independently identified articles eligible for in-depth examination using the same set of inclusion and exclusion criteria. Studies were included if either survival or acute toxicity related to radiation therapy were reported. Relevant radiation treatment modalities were defined as IMRT with or without comparison to 3D-CRT as the primary radiation technique performed as concurrent, neoadjuvant, or adjuvant therapy. We included cohort studies, case-control studies, cross-sectional surveys, and randomized controlled trials. When multiple articles reported on the same study population, we included only the studies with the largest population that met the inclusion criteria. We excluded case reports, case series, review articles, guidelines, commentaries, and editorials. Studies with only dosimetric or planning data were excluded. Studies not reporting survival outcome or toxicity grading were excluded. Patients receiving hypo-fractionated radiotherapy were excluded. We also excluded studies not published in English. Any discrepancies concerning article inclusion or exclusion between reviewers were resolved by a consensus meeting of three authors (WT Hsu, G Li and CN Chang). In the end, eight studies were included in this analysis (15-22).
Data extraction and synthesis
Data on study location, population characteristics including age range and sex ratio, number of participants, type of radiotherapy, chemotherapy, surgical interventions, resectability rate, median overall survival (OS), 1-year and 2-year OS were extracted. The incidence of acute GI and HEMA toxicity was calculated in each study with the available data. When studies were identified as containing pertinent data not included in the published article, we contacted the authors to obtain the missing data. When a response was not provided, such articles were excluded. When data regarding median OS, 1- or 2-year OS were not available in the text, we calculated the data from the reported Kaplan-Meier survival curves when available.
Quality assessment
The quality of the selected studies was evaluated independently by two reviewers using the modified Newcastle-Ottawa Scale (NOS). The NOS is a tool to assess the quality of non-randomized studies in terms of design, content, and ease of use directed to the task of incorporating the quality assessments. The NOS scale has eight domains: selection, including representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure, demonstration that the outcome of interest was not present at the start of the study; and comparability; outcome, including its assessment, follow-up period, and adequacy of follow up of cohorts (23). Disagreements between the two reviewers were resolved by a consensus discussion with a third author.
Statistical analysis
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for meta-analysis of observational studies in our data extraction, analysis, and reporting. Heterogeneity was tested using the Cochran Q statistic (P<0.05 where a P<0.05 suggested significant heterogeneity) and quantified with the I2 statistic, which describes the variation of effect size that is attributable to heterogeneity across studies (24,25). The value of the I2 statistic was used to select the appropriate pooling method: fixed-effects models were used for when I2 <50% and random-effects models for when I2 ≥50%. Confidence intervals (CIs) for the of I2 were calculated by the methods as suggested by Higgins et al (25,26). Pooled relative risks (RRs) were summarized with using the Mantel-Haenszel method for fixed-effects models (27) and the DerSimonian and Laird method for random-effects models (28). Statistical analyses were performed using the metan and metabias commands in Stata 11 (StataCorp, College Station, TX, USA). The metan, metabias, macros were used for meta-analytic procedures. P values <0.05 were considered statistically significant.
Results
Study characteristics
As shown in Figure 1, after screening of titles and abstracts, a total of 57 articles were identified for potential inclusion in our systematic review and meta-analysis. After examining the full texts of these articles, and applying our inclusion and exclusion criteria, we included eight cohort studies (with a total of 4,074 patients) in our final analysis, as shown in Table 1 (15-22). All studies were single-center cohort studies, except one study that analyzed the national cancer database jointly developed by the American College of Surgeons and the American Cancer Society (19). The large sample size of this study accounted for 65.9% (n=2,684) of all included patents. Most studies used a historical internal cohort of patients treated with 3D-CRT as the comparison group (16-22), except 2 studies (15,18) that used an external 3D-CRT cohort from a previously published randomized controlled trial as the comparison (29,30).
Table 1
| Study | Patient characteristics | Chemotherapy agents/radiation dose | Treatment modality | Sample size | Outcome | |
|---|---|---|---|---|---|---|
| IMRT group | 3D-CRT group | |||||
| Yovino S, 2011 | Pancreatic/ampullary cancer patients, post-operative or unresectable, T1–4, N0–2, M0 | Capecitabine or 5-FU/50.4 Gy | Adjuvant CRT, or definitive CRT | 497 | GI toxicities: 8.7% (4/46) | GI toxicities: 30.1% (136/451) |
| Adjuvant CRT | 482 | Median OS: 24.8 months | Median OS: 18.7 months | |||
| Definitive CRT | 15 | Median OS: 9.7 months | Median OS: NA | |||
| Definitive CRT | 15 | 1-year OS: 0% (0/15) | 1-year OS: NA | |||
| Definitive CRT | 15 | 2-year OS: 0% (0/15) | 2-year OS: NA | |||
| Adjuvant CRT | 482 | 1-year OS: 77.4% (24/31) | 1-year OS: 69.2% (312/451) | |||
| Adjuvant CRT | 482 | 2-year OS: 54.8% (17/31) | 2-year OS: 37.0% (167/451) | |||
| Combs SE, 2013 | Locally advanced pancreatic cancer, pre-operative | Gemcitabine/54 Gy (IMRT), 52.2 Gy (3D-CRT) | Neoadjuvant CRT with/without OP | 255 | Median OS: 11.0 months | Median OS: 12.3 months |
| 1-year OS: 36.8% (21/57) | 1-year OS: 46.0% (91/198) | |||||
| 2-year OS: 8.8% (5/57) | 2-year OS: 9.1% (18/198) | |||||
| Shaikh T, 2015 | Pancreatic cancer with stage any T, N0–1, M0, pre-operative | Gemcitabine/50.4 Gy | Neoadjuvant CRT with/without OP | 85 | HEMA toxicities: 46.6% (27/58) | HEMA toxicities: 33.3% (9/27) |
| Lee KJ, 2016 | Borderline resectable or locally advanced pancreatic cancer | Gemcitabine or 5-FU/50–62.5 Gy (IMRT), 45–50.4 Gy (3D-CRT) | Neoadjuvant CRT with/without OP | 84 | Median OS: 22.6 months | Median OS: 15.8 months |
| 1-year OS: 84.1% (37/44) | 1-year OS: 57.5% (23/40) | |||||
| 2-year OS: 59.1% (26/44) | 2-year OS: 20.0% (8/40) | |||||
| GI toxicities: 0% (0/44) | GI toxicities: 0% (0/40) | |||||
| Jin L, 2016 | Inoperable locally advanced pancreatic cancer | Gemcitabine/50.4 Gy | Definitive CRT | 234 | GI toxicities: 35.1% (34/97) | GI toxicities: 41.6% (57/137) |
| Prasad S, 2016 | Inoperable locally advanced pancreatic cancer | Gemcitabine or gemcitabine + 5-FU or capecitabine/56 Gy (IMRT), 50.4 Gy (3D-CRT) | Definitive CRT | 205 | Median OS: 15.3 months | Median OS: 15.3 months |
| 1-year OS: 61.2% (82/134) | 1-year OS: 62.0% (44/71) | |||||
| 2-year OS: 22.4% (30/134) | 2-year OS: 19.7% (14/71) | |||||
| GI toxicities: 5.2% (7/134) | GI toxicities: 2.8% (2/71) | |||||
| HEMA toxicities: 56.0% (75/134) | HEMA toxicities: 52.1% (37/71) | |||||
| Masui T, 2017 | Borderline resectable or locally advanced pancreatic cancer | Gemcitabine/42–45 Gy (IMRT), 39 Gy (3D-CRT) | Neoadjuvant CRT with/without OP | 30 | Median OS: 32.0 months | Median OS: 13.8 months |
| 1-year OS: 88.9% (16/18) | 1-year OS: 50.0% (6/12) | |||||
| 2-year OS: 61.1% (11/18) | 2-year OS: 16.7% (2/12) | |||||
| HEMA toxicities: 33.3% (6/18) | HEMA toxicities: 33.3% (4/12) | |||||
| Amini A, 2017 | Locally advanced pancreatic adenocarcinoma, T1–4, N0–1, M0 | Single or multiple agent(s)/45–54 Gy | Definitive CRT | 2,684 | Median OS: 12.0 months | Median OS: 11.0 months |
| 1-year OS: 49.1% (806/1,641) | 1-year OS: 45.2% (471/1,043) | |||||
| 2-year OS: 13.1% (215/1,641) | 2-year OS: 11.6% (121/1,043) | |||||
GI and HEMA toxicities ≥ grade 3 only. 3D-CRT, three-dimensional conformal radiotherapy; OS, overall survival; GI; gastrointestinal; HEMA, hematologic.
Quality assessment of the included studies
NOS was used to evaluate of the risk of bias and applicability, as shown is Table 2. There were three studies categorized as poor quality and high-risk of bias because comparability could not be confirmed (15,18,20). Nevertheless, the other five studies were categorized as good quality with low-risk of bias (16,17,19,21,22).
Table 2
| First author (country, year) | Selection component | Comparability | Outcome component | Total score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | |||
| Yovino S (US, 2011) | Somewhat representative of the average community ☆ | Drawn from a different source | Secure record ☆ | Yes ☆ | Difference in age and gender, multivariate analysis was not done | OS, GI toxicity ☆ | Yes ☆ | Yes ☆ | 6 (poor) |
| Combs SE (US, 2013) | Somewhat representative of the average community ☆ | Drawn from a different source | Secure record ☆ | Yes ☆ | Difference in age and gender, multivariate analysis was not done | OS ☆ | No description | Yes ☆ | 5 (poor) |
| Shaikh T (US, 2015) | Somewhat representative of the average community ☆ | Somewhat representative of the average community ☆ | Secure record ☆ | Yes ☆ | No difference in age, gender, location, staging and surgical intervention ☆ | Hematological toxicity☆ | Yes ☆ | Yes ☆ | 8 (good) |
| Lee KJ (Korea, 2016) | Somewhat representative of the average community ☆ | Somewhat representative of the average community ☆ | Secure record ☆ | Yes ☆ | No difference in age, gender, location, staging and surgical intervention ☆ | OS, GI toxicity ☆ | Yes ☆ | Yes ☆ | 8 (good) |
| Jin L (China, 2016) | Somewhat representative of the average community ☆ | Somewhat representative of the average community ☆ | Secure record ☆ | Yes ☆ | No specific description | GI toxicity ☆ | Yes ☆ | Yes ☆ | 7 (poor) |
| Prasad S (US, 2016) | Somewhat representative of the average community ☆ | Somewhat representative of the average community ☆ | Secure record ☆ | Yes ☆ | No difference in age, gender, location, staging and surgical intervention ☆ | OS, GI toxicity, hematologic toxicity ☆ | Yes ☆ | Yes ☆ | 8 (good) |
| Masui T (Japan, 2017) | Somewhat representative of the average community ☆ | Somewhat representative of the average community ☆ | Secure record ☆ | Yes ☆ | No difference in age, gender, location, staging and surgical intervention ☆ | OS, hematologic toxicity ☆ | Yes ☆ | Yes ☆ | 8 (good) |
| Amini A (US, 2017) | Somewhat representative of the average community ☆ | Somewhat representative of the average community ☆ | Secure record ☆ | Yes ☆ | Propensity score matching ☆ | OS ☆ | Yes ☆ | Yes ☆ | 8 (good) |
Abbreviation: Chemo, chemotherapy; RT, radiation therapy; OS, overall survival; GI, gastrointestinal; Gem, gemcitabine; Cape, capecitabine; NCDB, National Cancer Database.
Treatment modality and patient characteristics
Of the eight included studies, 3 included patients with advanced unresectable pancreatic cancer and used radiotherapy as definitive CRT (19,20,22), 4 studies included patients with locally advanced pancreatic cancer with borderline resectability and used radiotherapy as neoadjuvant therapy to achieve resectability (15-17,21), and the remaining study included a mixed patient population and used radiotherapy as either definitive or adjuvant therapy. Among four studies using radiotherapy as neoadjuvant treatment, the average rate of resectability after radiotherapy was 26.9% (range, 15.5–30.6%). Gemcitabine and 5-fluorouracil were the main chemotherapeutic agents used.
Dosimetric comparison of 3D-CRT and IMRT for organs at risk of toxicity
The radiation doses used ranged from 42.0 to 59.4 Gy. Only one study, Jin et al., reported the dosimetric comparison between IMRT and 3D-CRT in relation to toxicity; IMRT was associated with significantly reduced organ volumes at risk of toxicity at levels of 10, 20, or 30 Gy (20).
Treatment outcome comparison
Data on median OS, 1-year OS, and 2-year OS were available in six studies (15,16,18,19,21,22). The median OS of the IMRT group was 23.9 months (range, 11.0–32.0 months), as compared to 14.6 months (range, 11.0–18.7 months) in the 3D-CRT group. The 1-year OS ranged from 36% to 89%, and the 2-year OS ranged from 0% to 61%. Among the 4 studies of neoadjuvant CRT (15-17,21), Masui et al. reported the best outcome in patients receiving IMRT, with a median OS of 32.0 months, a 1-year OS of 89%, and a 2-year OS of 61%. In contrast, outcomes in patients receiving 3D-CRT were worse, with a median OS of 13.8 months, a 1-year OS of 50%, and a 2-year OS of 17%. In the single study that used postoperative adjuvant CRT as the main treatment modality (18), IMRT also showed a survival advantage over 3D-CRT; the median, 1-year, and 2-year OS for the IMRT group were 24.8 months, 77.4%, and 54.8%, as compared to 18.7 months, 69.2%, and 37.0% for 3D-CRT group, respectively.
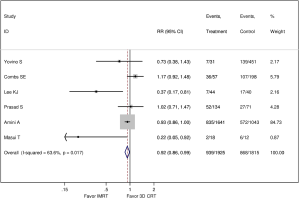
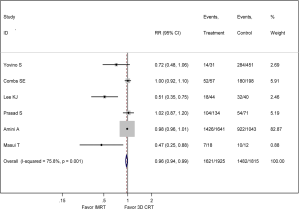
In the meta-analysis, six studies were available for comparison of IMRT and 3D-CRT regarding all-cause mortality (15,16,18,19,21,22), as shown in Figure 2 and Figure 3. The pooled RR comparing 1-year mortality between IMRT and 3D-CRT groups was 0.92 (95% CI: 0.86–0.99, P=0.017, I2 =63.6%; random-effect model). The pooled RR comparing 2-year mortality between IMRT and 3D-CRT groups was 0.96 (95% CI: 0.94–0.99, P=0.001, I2 =75.8%; random-effect model).
GI and HEMA toxicity
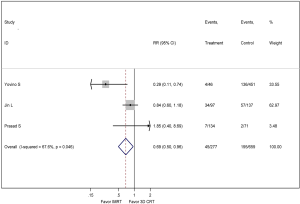
Data on GI toxicity were reported in four studies (n=1,020) (16,18,20,22) and data on HEMA toxicity were provided in three studies (n=320) (17,21,22). GI toxicity and HEMA toxicity were discriminated by NCI CTCAE version 4.0 (16,18,22), or NCI CTCAE version 3.0 (20). IMRT was associated with reduced GI toxicity but similar or higher HEMA toxicity as compared with 3D-CRT. The median incidence of ≥ grade 3 GI toxicity was 6.9% (range, 0–35.0%) in the IMRT group and 16.5% (range, 0–41.6%) in the 3D-CRT group. The median incidence of ≥ grade 3 HEMA toxicity was 46.6% (range, 33.3–56.0%) in the IMRT group and 33.3% (range, 33.3–52.1%) in the 3D-CRT group.
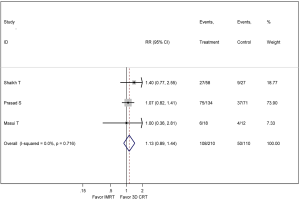
In the meta-analysis, three studies were available for comparison of IMRT and 3D-CRT regarding ≥ grade 3 GI toxicity (18,20,22), as shown in Figure 4. The pooled RR comparing ≥ grade 3 GI toxicity between IMRT and 3D-CRT groups was 0.69 (95% CI: 0.50–0.96, P=0.046, I2 =67.6%; random-effect model). Three studies were available for comparison of IMRT and 3D-CRT regarding ≥ grade 3 HEMA toxicity (17,21,22), as shown in Figure 5. IMRT did not significantly reduce ≥ grade 3 HEMA toxicity with a RR of 1.13 (95% CI: 0.89–1.44, P=0.716; fixed-effect model).
Discussion
IMRT is designed to more accurately deliver therapeutic radiation to target organs, thereby increasing dose delivery to tumors and reducing toxicity to normal organs. However, its effect on pancreatic cancer as compared with 3D-CRT has largely been inferred from case series without appropriate controls. Few parallel head-to-head comparisons of IMRT with 3D-CRT have been performed. Therefore, we conducted this systematic review and meta-analysis to summarize the current evidence regarding the effectiveness and toxicity of IMRT compared with 3D-CRT. We included 8 eligible studies, with a total of 4,074 patients, and found that IMRT was associated with improved survival and reduced GI or HEMA toxicity as compared with 3D-CRT. We did not find that the treatment modalities differed in terms of HEMA toxicity.
In comparison to a previous systematic review (31) that included several IMRT case series without appropriate controls, we only included studies with a parallel head-to-head comparison between IMRT and 3D-CRT. This strategy reduces the potential bias resulting from indirect comparisons, including heterogeneity in patient tumor staging, concurrent chemotherapy regimen, follow-up period, radiation dose, and quality of supportive care among different institutions. Furthermore, our systematic review and meta-analysis included 6 additional studies (15-17,19-22) not included in the previous review (31), reflecting a more contemporary approach to pancreatic cancer therapy.
Three of the 6 studies with outcome data showed a significant survival advantage of IMRT over 3D-CRT (16,19,21), likely on the basis of a higher dose delivered accurately to the target organ. In these 3 studies, the median radiation dose was higher in the IMRT group (42.0–58.4 Gy) than in the 3D-CRT group (39.0–47.7 Gy). The study by Masui et al. reported the best treatment outcomes, with a median survival of 32 months and a 2-year survival of 61% (21). In this study, the mean radiation dose in the IMRT group was 42.7 Gy, compared to 39.0 Gy in the 3D-CRT group. Other factors that may account for the better outcomes in this study include patients with less advanced pancreatic cancer, high resectability rate achieved (60%) after neoadjuvant therapy, and the use of S-1 treatment (oral form of Tegafur, gimeracil, and oteracil) after surgery (21). The study by Combs et al. reported the worst treatment outcomes, with a median OS of 11.0 months, a 1-year OS of 36%, and a 2-year OS of 8%. This study did not find a significant survival difference between IMRT and 3D-CRT (15). The poorer outcomes in this study may be due to the inclusion of more advanced stage pancreatic cancer and the low resectability rate (30%) achieved after neoadjuvant therapy (15). In meta-analysis, IMRT still significantly reduced mortality, not just reduced the dose to organs at risk or radiation toxicity. For eliminating the diversities of different study group and setting the appropriate weights to studies with small sample size, Mantel-Haenszel weight and DerSimonian and Laird method were utilized (27,28). We can define this finding as more accurate compared with previous systematic review.
Among studies using IMRT as definitive CRT, the study by Prasad et al. showed the best prognosis, with a median OS of 15.3 months, a 1-year OS of 61%, and a 2-year OS of 22%. The better outcome in this study may be explained by the use of gemcitabine-based treatment regimens in many patients (56%) and the higher irradiation dose delivered (56.0 Gy) (22). The study by Amini et al. showed a median OS of 12.0 months, a 1-year OS of 49%, and a 2-year OS of 13% in the IMRT group. The treatment outcomes in the 3D-CRT group were inferior, with a median OS of 11.0 months, a 1-year OS of 45%, and a 2-year OS of 12% (19).
Although this study has the largest number of patients, the study was conducted at a time when gemcitabine was not widely used in patients with pancreatic cancer. Previously, patients who underwent definitive CRT own the highest severity and the worst prognosis, median OS was around 11.2 months in ECOG E4201 trial, included patients who underwent definitive CRT with 3D-CRT (32). These studies we selected in our systematic review and meta-analysis reported the better survival compared with the outcome of ECOG E4201 trial, published on 2011.
In regard to radiation toxicity, most studies showed that IMRT resulted in a lower incidence of GI complications as compared to 3D-CRT; however, the study by Jin et al. did not find a significant difference between IMRT (35%) and 3D-CRT (42%) (20).
In meta-analysis, IMRT reduced significantly ≥ grade 3 GI toxicity, as our expectation. Previously, we usually think of IMRT as the technique reducing doses to organs at risk, nevertheless, we can confirm IMRT is able to reduce the GI toxicity now. In relation to HEMA toxicity, IMRT was not associated with a lower incidence of complications as compared to 3D-CRT. The study by Prasad et al. reported the highest incidence of HEMA toxicity, with an incidence of 56% in the IMRT group and 52% in the 3D-CRT group (22). The study by Masui et al., however, reported the lowest incidence of HEMA complications (33%) in both groups (21). The lack of a bone marrow-sparing effect of IMRT may be explained by the widespread use of concurrent gemcitabine chemotherapy in this group, which may have resulted in more myelosuppression (33). Since gemcitabine is the conventional and effective chemotherapy regimen, we cannot cut off it only for HEMA toxicity. All we can do are monitoring lab data of hematology and preventing infection carefully.
Limitations
Results of this systematic review and meta-analysis should be interpreted in view of some limitations. First, the survival benefit of IMRT may be exaggerated because of the differences in clinical practice over time. Amini et al. analyzed the US national cancer database and showed that the use of IMRT increased from 50% in 2005 to 80% in 2006, with a persistent increasing trend thereafter (19). When 3D-CRT was the prevalent treatment modality, the efficacy of the chemotherapeutic agent gemcitabine was less clear, and it was thus utilized less often. We found that patients receiving IMRT were more likely to receive gemcitabine-based concurrent chemotherapy (16,22); therefore, the survival advantage of IMRT may also be related to the chemotherapeutic regimen used. Advancements in chemotherapy, targeted therapy, and supportive care also need to be taken into account. Secondly, contemporary head-to-head comparison studies are limited; thus, we were unable to perform a potential publication bias analysis. Lastly, Amini et al. is the study based on NCDB, so it consists massive study population, compared with other studies of smaller sample size. The fact that Amini et al. accounts for the dominate group of all included patients, may affect the outcome.
Conclusions
Our systematic review and meta-analysis suggests that IMRT may be superior to 3D-CRT in the treatment of pancreatic cancer and may be associated with significantly reduced incidence of GI toxicity. We did not observe a reduction in the incidence of HEMA toxicity with IMRT. IMRT may be considered as a standard curative treatment for patients with pancreatic cancer, regardless of patient characteristic or treatment modality.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2018.07.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Malik NK, May KS, Chandrasekhar R, et al. Treatment of locally advanced unresectable pancreatic cancer: a 10-year experience. J Gastrointest Oncol 2012;3:326-34. [PubMed]
- Gillen S, Schuster T, Meyer Zum Buschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267 [Crossref] [PubMed]
- Nakamura A, Shibuya K, Matsuo Y, et al. Analysis of dosimetric parameters associated with acute gastrointestinal toxicity and upper gastrointestinal bleeding in locally advanced pancreatic cancer patients treated with gemcitabine-based concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys 2012;84:369-75. [Crossref] [PubMed]
- Taremi M, Ringash J, Dawson LA. Upper abdominal malignancies: intensity-modulated radiation therapy. Front Radiat Ther Oncol 2007;40:272-88. [Crossref] [PubMed]
- Lee KJ, Kim HM, Jung JW, et al. Gastrointestinal hemorrhage after concurrent chemoradiotherapy in locally advanced pancreatic cancer. Gut Liver 2013;7:106-11. [Crossref] [PubMed]
- Nutting C, Dearnaley DP, Webb S. Intensity modulated radiation therapy: a clinical review. Br J Radiol 2000;73:459-69. [Crossref] [PubMed]
- Cheung K. Intensity modulated radiotherapy: advantages, limitations and future developments. Biomed Imaging Interv J 2006;2:e19 [Crossref] [PubMed]
- Bai YR, Wu GH, Guo WJ, et al. Intensity modulated radiation therapy and chemotherapy for locally advanced pancreatic cancer: results of feasibility study. World J Gastroenterol 2003;9:2561-4. [Crossref] [PubMed]
- Ben-Josef E, Schipper M, Francis IR, et al. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 2012;84:1166-71. [Crossref] [PubMed]
- Pipas JM, Zaki BI, McGowan MM, et al. Neoadjuvant cetuximab, twice-weekly gemcitabine, and intensity-modulated radiotherapy (IMRT) in patients with pancreatic adenocarcinoma. Ann Oncol 2012;23:2820-7. [Crossref] [PubMed]
- Zhong J, Patel K, Switchenko J, et al. Outcomes for patients with locally advanced pancreatic adenocarcinoma treated with stereotactic body radiation therapy versus conventionally fractionated radiation. Cancer 2017;123:3486-93. [Crossref] [PubMed]
- Golden DW, Novak CJ, Minsky BD, Liauw SL. Radiation dose ≥54 Gy and CA 19-9 response are associated with improved survival for unresectable, non-metastatic pancreatic cancer treated with chemoradiation. Radiat Oncol 2012;7:156. [Crossref] [PubMed]
- Kharofa J, Tsai S, Kelly T, et al. Neoadjuvant chemoradiation with IMRT in resectable and borderline resectable pancreatic cancer. Radiother Oncol 2014;113:41-6. [Crossref] [PubMed]
- Tunceroglu A, Park JH, Balasubramanian S, et al. Dose-painted intensity modulated radiation therapy improves local control for locally advanced pancreas cancer. ISRN Oncol 2012;2012:572342 [Crossref] [PubMed]
- Combs SE, Habermehl D, Kessel K, et al. Intensity modulated radiotherapy as neoadjuvant chemoradiation for the treatment of patients with locally advanced pancreatic cancer. Outcome analysis and comparison with a 3D-treated patient cohort. Strahlenther Onkol 2013;189:738-44. [Crossref] [PubMed]
- Lee KJ, Yoon HI, Chung MJ, et al. A Comparison of Gastrointestinal Toxicities between Intensity-Modulated Radiotherapy and Three-Dimensional Conformal Radiotherapy for Pancreatic Cancer. Gut Liver 2016;10:303-9. [Crossref] [PubMed]
- Shaikh T, Wang LS, Egleston B, et al. Predictors of Hematologic Toxicity and Chemotherapy Dose Intensity in Patients Undergoing Chemoradiation for Pancreatic Cancer. Am J Clin Oncol 2018;41:59-64. [PubMed]
- Yovino S, Poppe M, Jabbour S, et al. Intensity-modulated radiation therapy significantly improves acute gastrointestinal toxicity in pancreatic and ampullary cancers. Int J Radiat Oncol Biol Phys 2011;79:158-62. [Crossref] [PubMed]
- Amini A, Jones BL, Stumpf P, et al. Patterns of Care for Locally Advanced Pancreatic Adenocarcinoma Using the National Cancer Database. Pancreas 2017;46:904-12. [Crossref] [PubMed]
- Jin L, Wang R, Jiang S, et al. Dosimetric and clinical toxicity comparison of critical organ preservation with three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, and RapidArc for the treatment of locally advanced cancer of the pancreatic head. Curr Oncol 2016;23:e41-8. [Crossref] [PubMed]
- Masui T, Takaori K, Anazawa T, et al. A Prospective Study of Intensity-modified Radiation Therapy in Comparison with Conventional 3D-RT for BR Pancreatic Cancer Patients with Arterial Involvement. Anticancer Res 2017;37:7023-30. [PubMed]
- Prasad S, Cambridge L, Huguet F, et al. Intensity modulated radiation therapy reduces gastrointestinal toxicity in locally advanced pancreas cancer. Pract Radiat Oncol 2016;6:78-85. [Crossref] [PubMed]
- Deeks JJ, Dinnes J, D'Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7:iii-x, 1-173. [Crossref] [PubMed]
- Cochran WG. The comparison of percentages in matched samples. Biometrika. 1950;37:256-66. [Crossref] [PubMed]
- Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [Crossref] [PubMed]
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719-48. [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet 2001;358:1576-85. [Crossref] [PubMed]
- Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 2008;299:1019-26. [Crossref] [PubMed]
- Bittner MI, Grosu AL, Brunner TB. Comparison of toxicity after IMRT and 3D-conformal radiotherapy for patients with pancreatic cancer - a systematic review. Radiother Oncol 2015;114:117-21. [Crossref] [PubMed]
- Loehrer PJ Sr, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol 2011;29:4105-12. [Crossref] [PubMed]
- Barton-Burke M. Gemcitabine: a pharmacologic and clinical overview. Cancer Nurs 1999;22:176-83. [Crossref] [PubMed]
Cite this article as: Chang CN, Hsu WT, Li G. Effectiveness and acute toxicity of intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy in pancreatic cancer: a systematic review and a meta-analysis. Ther Radiol Oncol 2018;2:30.