The survival impact of multimodal therapy in Masaoka stage IV thymic tumors
Introduction
The thymic tumor is the most frequent anterior mediastinal tumor and constitutes 15–30% of all mediastinal tumors. The histologic types are classified under thymoma, thymic carcinoma, and thymic carcinoid. Thymic carcinoma has the worse prognosis among thymomas due to its extensive locoregional invasion, highly-disseminated distant metastasis, and aggressive clinical course. Masaoka staging system is the one most widely used to classify stages of thymic neoplasms (1). According to this staging system, stage IV diseases are further divided into two finer categories: IVa and IVb. The IVa disease has pleural or pericardial dissemination, and the IVb disease has lymphogenous or hematogenous metastasis. For Masaoka stage IV thymic malignancy regardless of histologic types, the 5-year overall survival (OS) rate falls between 47% and 80% (1-3). Kondo et al. (4) reported for the Japanese patients with thymic carcinomas, the 5-year survival rates as follows: stage I/II: 88.2%, stage III: 51.7%, and stage IV: 37.6%. Due to the rarity of these tumors, the prognostic factors and efficacy of treatment modalities remain largely unclear. Apart from the descriptive classification based on histology, the prognostic correlation was also investigated. For example, low-grade diseases have been reported to lead a more favorable clinical course (median survival of 25.4 months to >6.6 years) compared with those of high-grade (median survival of 11.3 to 15.0 months) (5). Complete resection was reported in several studies to be the critical factor of survival outcome. Nevertheless, it is unclear if the debulking surgery is also effective in extending survival or to improve the disease control in advanced or high-grade malignancies (4,6). The optimal strategy of adjuvant therapy therefore remains controversial. Radiotherapy may be beneficial in local control and survival in selected groups of patients, such as those with macroscopic adherence to the pleura (7). Chemotherapy has been used in the induction therapy (8), concurrently with radiotherapy for locally advanced tumors (9), or adjuvant regimen for the advanced disease (10). The purpose of this study is to retrospectively determine the role of multimodal therapy for Masaoka stage IV thymic tumors in terms of survival outcomes and prognostic variables.
Methods
Patients
We enrolled 26 patients with histologically proven Masaoka stage IV thymic malignancies between Aug 2006 and December 2014. In 22 operable cases, treatment was surgical resection and postoperative irradiation, followed by adjuvant cisplatin-based chemotherapy. The remaining four cases were inoperable, and the patients received treatment that was of individualized strategy. Specifically, one patient received radiotherapy alone; one received neoadjuvant chemotherapy alone (and lost follow-up after completion of chemotherapy); one received definite concurrent chemoradiotherapy (CCRT); and one received neoadjuvant chemotherapy followed by CCRT, thereafter adjuvant-treatment with 5-fluorouracil. All patients received complete cancer staging surveys which included history taking, physical examinations, laboratory tests, thoracic imaging [computed tomography (CT) and/or magnetic resonance imaging (MRI)], ultrasound scanning of the abdomen, and a whole-body bone scan. All patients signed the informed consent of treatment (surgery, chemotherapy, and radiotherapy) before initiation of multimodal therapy. A retrospective analysis was conducted by reviewing medical records, pathology database, and electronic imaging results.
Surgery
For the 22 operable patients, the initial management was surgical resection, which definitively established the diagnosis and accurately evaluated the locoregional extent of the disease. Under general anesthesia with double-lumen endotracheal tube, the patient was put in supine position. The operation field was sterilized and draped as usual aseptic procedure. Midline incision over anterior chest was done and median sternotomy was thereafter performed from sternal notch to xiphoid process. Mediastinum was explored up to the cervical thymic extensions and laterally down to the phrenic nerves. Extended resection of thymic tumor and perithymic fat was performed with the intention to achieve macroscopically complete resections. If the gross disease involved pericardium, pleura, great vessels, chest wall, diaphragm, neck lymphadenopathies, and lung parenchyma, debulking surgery was then performed whenever feasible. If cardiopulmonary bypass support was required, the ascending aorta and the femoral vein were cannulated. If necessary, the pericardium defect was repaired with artificial pericardium patch. Dissection of mediastinal and hilar lymph nodes was performed if grossly hard, the enlarged nodal disease had been noted. If needed, an otorhinolaryngologist was consulted for neck lymphadenopathies.
Pathological analyses
Pathological examinations included histologic typing, tumor extension, distribution of metastatic lymphadenopathies, and resection margins. Histologic classification was listed in Table 1. We found nine patients with stage IVa disease (pleural dissemination in 6, pericardial dissemination in 3); 12 patients with stage IVb disease (seven patients had lymphogenous metastasis, three patients had hematogenous metastasis, two patients had both lymphogenous and hematogenous metastasis); and five patients with stage IVa + IVb disease.
Table 1
| Histologic classification | Number |
|---|---|
| Thymic carcinoma: 17 patients | |
| Undifferentiated carcinoma | 4 |
| Neuroendocrine carcinoma | 3 |
| Lymphoepithelioma-like, high grade | 1 |
| Squamous cell carcinoma, poorly differentiated | 1 |
| Thymic carcinoma, NOS | 7 |
| Malignant solitary fibrous tumor | 1 |
| Thymoma: 9 patients | |
| WHO type B1 thymoma | 2 |
| WHO type B2 thymoma | 3 |
| WHO type B3 thymoma | 3 |
| WHO type B thymoma, NOS | 1 |
NOS, not otherwise specified.
Radiotherapy
The radiation therapy was planned by intensity-modulated radiotherapy (IMRT) treatment planning. All patients underwent CT simulation in a supine position with arms placed above the head. A customized vacuum bag was used for immobilization. CT images were taken at 5-mm intervals covering the region of neck and thorax. On the CT images, the followings were outlined: gross tumor volume (GTV), clinical target volume (CTV), planning target volume (PTV) and organs at risk (OARs). GTV was defined as grossly identified regions of disease. In accordance with the pre-operative thoracic imaging, CTV was delineated from the GTV and postoperative tumor bed plus 5-mm margin, with the involved station of mediastinal lymphadenopathies. PTV was defined as CTV plus 5-mm margin to account for daily setup error and internal organ motion. The IMRT based on the multiple field technique was delivered through a linear accelerator (Varian 2100EX with a 120-leaf Millennium multileaf collimator, Varian Oncology Systems, Palo Alto, CA, USA) using 6 MV photons. Doses were calculated through the Varian Eclipse planning system (versions 6.5 to 7.2.24) (Varian Medical Systems Inc., Worldwide Headquarters 3100 Hansen Way, Palo Alto, CA94304, USA) based on the pencil beam model. The radiation dose was delivered to PTV at 1.8 to 2 Gy per fraction per day and 5 days per week. Radiation dose was escalated to 60 to 66 Gy if the gross disease had been noted. Total radiation doses of patients ranged from 50 to 66 Gy. Radiation dose was prescribed such that its 95% of the PTV would receive 100% of the prescribed dose. The dose constraints for OARs were <18 Gy for mean lung dose, <20% lung volume received >20 Gy (V20), and <67% heart volume received >45 Gy (V45), and <50 Gy for the entire spinal cord.
Chemotherapy
Chemotherapy regimens were mostly cisplatin-based. In the operable patients, adjuvant chemotherapy was given with CAP regimen from 2006 to 2010, and PE regimen since 2010. CAP regimen was given triweekly for 6 courses with cisplatin 50 mg/m2 on day 1, doxorubicin 50 mg/m2 on day 1, cyclophosphamide 500 mg/m2 on day 1. PE regimen was given triweekly for 6 courses with cisplatin 60 mg/m2 on day 1, etoposide 100 mg/m2 on day 1 to 3. Patients who were not suitable for the operation were shifted to definite CCRT with or without neoadjuvant and/or adjuvant chemotherapy. During the course of radiation, chemotherapy was given concomitantly with mitomycin 5 mg/m2 on day 1, followed by weekly cisplatin 30 mg/m2 on day 8, 15, 22, 29. Pre-chemotherapy evaluations were performed every time before chemotherapy, including history taking, vital sign, white blood count, hemoglobin, platelet, liver function, renal functions, chest X-ray, and urine analysis. Chemotherapy was administered only when the patient had displayed no signs of infection, and with absolute neutrophil count (ANC) >1,500/µL, Hb >10 g/dL, platelet >100,000/µL, as well as normal liver and renal functions.
Follow-up and survival
During the first three post-treatment years, at intervals of 3–4 months, patients underwent follow-up surveys that included chest CT scan, bone scan, liver sonography. In the following years, restaging was performed every 6 months up to end of 5 years post-treatment. At each hospital visit, the patient was evaluated to assess late toxicities and disease recurrence. Mortality cases were documented. The severity of toxicity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.3.
Statistical analyses
The Kaplan-Meier method was used to analyze survival, and comparisons were done with the log-rank test. The Cox proportional hazards regression model was used to estimate the 95% confidence intervals. P values <0.05 were considered statistically significant. Statistical analyses were performed with the SPSS software, version 19.0 (SPSS).
Results
Patient characteristics
Patient characteristics are listed in Table 2. The median follow-up time of survivors was 63.32 months. Seventeen patients had thymic carcinomas, and 9 had WHO type B thymomas. Between the groups with thymic carcinoma and with thymoma, we found no statistically significant differences in age, sex, or disease stage.
Table 2
| Characteristic | Thymic carcinoma (n=17) | Thymoma (n=9) | Total (n=26) |
|---|---|---|---|
| Age, median [range], years | 50 [18–77] | 43 [32–51] | 48 [18–77] |
| Male/female, n | 12/5 | 3/6 | 15/11 |
| Stage, n [%] | |||
| IVa | 5 [29] | 4 [44] | 9 [35] |
| IVb | 12 [71] | 5 [56] | 17 [65] |
| Treatment modality, n [%] | |||
| Surgery | 13 [76] | 9 [100] | 22 [85] |
| Radiotherapy | 16 [94] | 9 [100] | 25 [96] |
| Chemotherapy | 12 [71] | 6 [67] | 18 [69] |
Survival outcomes
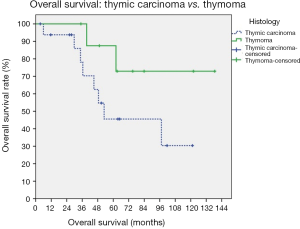
In all patients (n=26), the survival rates were: 69.2% for 5-year OS, 38.5% for progression-free survival, 57.7% for locoregional recurrence-free survival, and 42.3% for distant progression-free survival. The median survival was 49.98 months (range, 5.93–138.57 months) and the median progression-free survival was 28.83 months (range, 2.33–121.33 months). In those with thymic carcinomas (n=17 patients), the survival rates were 58.8% for 5-year OS, 41.2% for progression-free survival, 58.8% for locoregional recurrence-free survival, and 47.1% for distant progression-free survival. Their median survival was 45.07 months (range, 3.53–121.33 months) and the median progression-free survival was 16.73 months (range, 2.33–121.33 months).
Prognostic factors
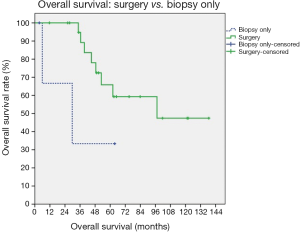
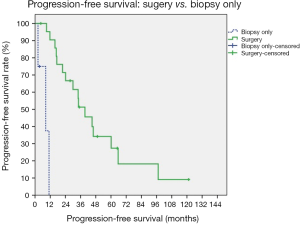
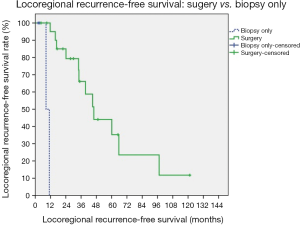
Survival analyses of prognostic factors are listed in Figures 1-4. Using the univariate analysis, we evaluated the following prognostic factors: the extent of resection (surgery vs. biopsy only), Masaoka staging (IVa vs. IVb), and pathology (thymic carcinoma vs. thymoma). For survivals of progression-free and locoregional recurrence-free conditions, the statistically significant predictor was surgical intervention (surgery vs. biopsy only, P<0.001 for the above two conditions). The 5-year progression-free survival rates for patients undergoing surgery was 41%, and biopsy alone 25% (P<0.001). Biopsy alone and thymic carcinoma had trends toward poorer OS, although these were not statistically significant.
Discussion
In the present study, 5-year OS rates were 69.2% in 26 patients with stage IV thymic tumors, and 58.8% in 17 patients with stage IV thymic carcinomas. Our results appeared better than those reported by Kondo et al. (4) in the Japanese population (i.e., 37.6% for 5-year OS in patients with stage IV thymic carcinomas).
Since surgery is the mainstay of the multimodal therapy, complete resection should always be considered. However, some patients in stage IV diseases are inoperable. In stage III–IV diseases, Hishida et al. (11) showed that R1 and R2 sub-total resection groups with similar OS curves (P=0.955). Results were superior to that of R2 non-resection group. For Masaoka stage IVa disease with dissemination, the 5-year OS rates after R1 and R2 sub-total resection were 50% and 45%, better than after R2 non-resection (6 patients, OS: 0%). Besides, complete resection in stage IV diseases still achieved relatively favorable recurrence-free survival and OS, which were similar to stage III diseases. In the present study, surgical resection (including debulking surgery) is the key factor for better progression-free survival and locoregional control. In summary, the maximal debulking surgery might be beneficial for advanced diseases.
In the present study, radiation planning adopted the involved-field technique. Kondo et al. (4) reported that prophylactic mediastinal radiotherapy does not effectively prevent local recurrences in patients with totally resected stage II and III thymomas. Exact indications for radiation are unclear. Adjuvant radiotherapy with precise focusing on high-risk region should be considered if complete resection is not feasible. In 25 patients received radiotherapy as part of multimodal therapy, most of them fulfilled V20 Gy <20% (5–10%: 3 patients; 10–15%: 1 patient; 15–20%: 19 patients; 35–40%: 2 patients). Only 8% (2 of 25 patients) violated the ideal constraint. One patient received induction chemotherapy with the persistently bulky disease, followed by definite CCRT. The other patient received neoadjuvant chemotherapy and debulking surgery with R2 resection (residual disseminated tumors over lower part pleura, diaphragm, and left lower lung surface), followed by postoperative left hemithoracic radiation. Due to relatively smaller RT field (postoperative tumor bed without prophylactic mediastinal radiation, and PTV margin only 5 mm) and IMRT technique, 92% of 25 patients fulfilled V20Gy <20%. There would be a balance between locoregional control and toxicities. In this study, the majority of the first failure was the distant metastasis. It seemed that locoregional control not to be compromised with the relatively smaller RT filed. To reduce radiation-related toxicities, the use of gating techniques in minimizing respiratory variations may contribute to highly-conformal dose distribution in silico. For clinical practice in the real world, given the poor performance status in stage IV diseases, the prolonged treatment in time should be another factor to consider in planning the strategy. Volumetric-modulated arc therapy (VMAT) may be a good alternative technique in terms of a comparable dose distribution with a shorter treatment time. Since the development of RT techniques over the last few decades, the escalation of radiation doses (to gross disease to 60–66 Gy) as in this study, was safely delivered by the VMAT technique without violating RT dose constraints for OARs.
For advanced thymic malignancies, chemotherapy can be used in the induction therapy (8), concurrent setting with radiotherapy for locally advanced tumors (9), or adjuvant regimen for advanced disease (10). Some studies have revealed that one of the benefits of induction chemotherapy is to convert unresectable diseases to resectable (12). In this study, one of the inoperable patients received neoadjuvant chemotherapy with cisplatin and etoposide. Due to the significant relief in symptoms after chemotherapy, this patient was hesitated to receive further local therapy and even dropped out from our follow-up. Therefore, upfront debulking surgery was favored in our institution to maximally eradicated local diseases, even though complete resection of locoregional disease could not achieve in some patients [R1 resection: 36% (8/22); R2 resection: 5% (1/22)]. In addition, upfront debulking surgery may provide additional information for pathological staging and clinical targets of postoperative radiation therapy such as close/positive margin(s), capsular invasion, and residual pleural or pericardial nodule(s). For physicians, it is essential to thoroughly inform patients about the treatment planning, especially those who may be prescribed with neoadjuvant therapy.
Conclusions
Multimodality therapy improved survival outcomes for stage IV thymic malignancies. Surgical resection, including debulking surgery prior to other adjuvant treatments, is the key factor for improving progression-free survival and local control for patients even including those with stage IV diseases.
Acknowledgments
Language editing assistance: Dr. Paul W. F. Poon, Ph.D. in Neural Sciences from the Indiana University of USA.
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2018.06.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of the Taichung Veterans General Hospital (No. CE17055A) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Nicholson AG, Detterbeck FC, Marino M, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the T Component for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S73-80. [Crossref] [PubMed]
- Kondo K, Van Schil P, Detterbeck FC, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the N and M components for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S81-7. [Crossref] [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. [Crossref] [PubMed]
- Suster S, Rosai J. Thymic carcinoma. A clinicopathologic study of 60 cases. Cancer 1991;67:1025-32. [Crossref] [PubMed]
- Liu HC, Chen YJ, Tzen CY, et al. Debulking surgery for advanced thymoma. Eur J Surg Oncol 2006;32:1000-5. [Crossref] [PubMed]
- Haniuda M, Miyazawa M, Yoshida K, et al. Is postoperative radiotherapy for thymoma effective? Ann Surg 1996;224:219-24. [Crossref] [PubMed]
- Kawasaki H, Taira N, Ichi T, et al. Weekly chemotherapy with cisplatin, vincristine, doxorubicin, and etoposide followed by surgery for thymic carcinoma. Eur J Surg Oncol 2014;40:1151-5. [Crossref] [PubMed]
- Korst RJ, Bezjak A, Blackmon S, et al. Neoadjuvant chemoradiotherapy for locally advanced thymic tumors: a phase II, multi-institutional clinical trial. J Thorac Cardiovasc Surg 2014;147:36-44. [Crossref] [PubMed]
- Kim BK, Cho BC, Choi HJ, et al. A single institutional experience of surgically resected thymic epithelial tumors over 10 years: clinical outcomes and clinicopathologic features. Oncol Rep 2008;19:1525-31. [PubMed]
- Hishida T, Nomura S, Yano M, et al. Long-term outcome and prognostic factors of surgically treated thymic carcinoma: results of 306 cases from a Japanese Nationwide Database Study. Eur J Cardiothorac Surg 2016;49:835-41. [Crossref] [PubMed]
- Kim ES, Putnam JB, Komaki R, et al. Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report. Lung Cancer 2004;44:369-79. [Crossref] [PubMed]
Cite this article as: Li C, Yeh HL. The survival impact of multimodal therapy in Masaoka stage IV thymic tumors. Ther Radiol Oncol 2018;2:25.