
The impacts of chemotherapeutic response for clinical stage II and III breast cancer patients after neoadjuvant chemotherapy
Introduction
Breast cancer is the most common malignancy among women in Taiwan. According to the 2014 cancer registry annual report in Taiwan, 11,769 new cases of invasive breast cancer were diagnosed in women, and 2,071 women died from the disease (1). Due to the heterogeneous nature of breast cancer, there are different therapeutic modalities used for invasive breast cancer patients. Neoadjuvant chemotherapy (NAC) is the standard of care for patients with locally advanced breast cancer, and there has been an increase in the utilization of neoadjuvant treatment for the management of invasive breast cancer over the last decade (2). Although previous randomized clinical trials have demonstrated no survival differences between neoadjuvant and adjuvant chemotherapy, NAC can provide opportunities for undergoing breast conserving surgery (BCS) and assessing tumor response to chemotherapy, as well as for adapting the regimens of adjuvant chemotherapy accordingly (3,4).
Roughly 25% of all breast cancers overexpress the human epidermal growth factor receptor 2 (HER2/neu) (5). Trastuzumab, a humanized monoclonal antibody, suppresses the proliferation, growth, and survival of cancer cells with HER2/neu overexpression via direct and indirect mechanisms (6). The NOAH trial and other studies confirmed that trastuzumab had survival benefits in the treatment of patients with early operable HER2/neu-positive breast cancer, when combined with chemotherapy and followed by surgery and adjuvant radiotherapy. In addition, trastuzumab patients also had significant improvements of pathologically complete responses (CRs) and significantly decreased risks of disease progression, relapse, and death, compared with the chemotherapy-only patients (7). Thus, most studies have focused their attention on pathological complete response (pCR) as the primary endpoint for responses to NAC.
Based on the National Comprehensive Cancer Network (NCCN) guideline for invasive breast cancer, version 1.2017, adjuvant radiotherapy is recommended for patients with cT3-4, cN2-3, or stage III disease (8). In addition, a previous review article showed that, in the patients that were clinically node-negative before NAC and pathologically node-negative after NAC, omission of adjuvant regional nodal irradiation (RNI) did not result in an increased risk of regional failure or decreased overall survival (OS) (9). However, the role of adjuvant RNI is still unresolved for patients with clinically node-positive early stage disease, who then become pathologically node-negative after NAC.
In this retrospective study, we divided patients into two groups, responders and non-responders, according to their radiologic and pathological responses to NAC, and investigated the possible prognostic factors of responders and non-responders. Moreover, we also reviewed the rate of pCR in patients with clinical stage II/III invasive breast cancer who were treated at Changhua Christian Hospital. Furthermore, we intended to identify the role of adjuvant RNI in the patients with a pathologically node-negative status using a subgroup analysis.
Methods
We analyzed a cohort of 133 clinical stage II/III patients with invasive breast cancer who were treated at our institute from April 2007 to December 2012. Only unilateral, non-recurrent, non-metastatic tumors with histologic type invasive ductal carcinoma were included. All patients received NAC, followed by surgery and radiotherapy. The study was conducted according to the ethical and institutional rules concerning research on patients and was approved by the breast cancer multidiscipline group of Changhua Christian Hospital.
All clinical diagnoses of patients included core needle biopsy confirmation. The following clinical features were collected prior to NAC: age at diagnosis, histologic type, initial tumor size, tumor location, nodal status, tumor grade, and biomarker status, such as estrogen receptor (ER), progesterone receptor (PgR), and HER2/neu status. Initial tumor size and nodal status were recorded by either breast MRI, mammography, or breast sonography and were assessed by certified radiologists and surgical oncologists. Pathological features after NAC were also retrieved, including histologic type, tumor size, nodal status, tumor grade, ER and PgR status, HER2/neu status, number of metastatic nodes, extranodal extension (ENE), lymphovascular invasion (LVI), surgical margin, and total numbers of sentinel and non-sentinel nodes.
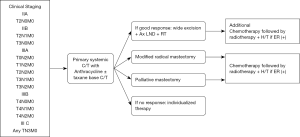
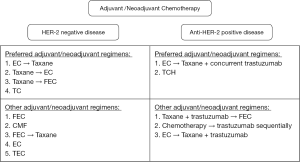
All patients were treated according to the clinical practice guidelines of the institute (Figure 1). The NAC regimens included anthracycline-based regimens, taxane-based regimens, or anthracycline-taxane regimens, and decisions were made at the physician’s discretion (Figure 2). One year of treatment with trastuzumab, given as neoadjuvant and adjuvant treatments, was prescribed to patients with HER2/neu overexpression or amplification. Endocrine therapies (tamoxifen, aromatase inhibitor, or GnRH agonists) were prescribed when indicated. Surgery was performed 2–6 weeks after the end of NAC and all patients received adjuvant radiotherapy. In cases of BCS, a total dose of 45–50.4 Gy with a daily fraction size of 1.8–2.0 Gy was delivered to the breast, with or without the ipsilateral regional nodal areas, followed by a 10–16 Gy boost to the tumor bed. Chest wall radiation consisting of 50–50.4 Gy (1.8 to 2.0 Gy per fraction), with or without RNI, was followed by a 10–16 Gy boost to the tumor bed, administered after mastectomy. Indications of RNI included pathologically positive nodal status or pathologically negative nodal status with primary tumor ≥5 cm. With regards to administration of the tumor bed boost, patients at high risk of local recurrence (age <50, LVI, close margin, and positive nodal status) were recommended. However, final decisions for RNI and the tumor bed boost were at the physicians’s discretion. Moreover, adjuvant chemotherapy was administered according to clinicopathological and tumor biological variables (Figure 2).
For the purpose of defining tumors that would not benefit from NAC, the cohort of patients was divided into two groups, responders and non-responders, according to their radiological and pathological responses to NAC. Assessment of initial tumor size was defined as the longest diameter obtained from the imaging techniques performed. We compared the initial clinical tumor size with the size of the pathological residual tumor after NAC and used the Response Evaluation Criteria in Solid Tumor (RECIST 1.1) as a reference (10). In our study, responders, including those with a CR or partial response (PR), were defined with following criteria. The response was considered complete if there was no histologic evidence of residual invasive tumor in all resected specimens of the breast and axillary lymph nodes (ypT0/is, ypN0). A PR was defined as a minimum 30% decrease in the maximal diameter of the residual tumor pathologically compared with that found clinically with no signs of progression in any lesions, or as assessed by the pathologist (if available). Those who did not fulfil the criteria for CR and PR were classified as non-responders.
Survival time was estimated using the Kaplan-Meier method; Chi-squared or Fisher’s exact tests were used for univariate analyses, and multivariate Cox models were performed to estimate the HR and 95% CI. A P value of 0.05 or less was considered to indicate statistical significance. Statistical analyses were performed using the IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA).
Results
One hundred and thirty-three patients with clinical stage II/III invasive ductal carcinoma received anthracycline- or taxane-based NAC with or without targeted therapy, followed by curative surgery and adjuvant radiotherapy. The median follow-up time was 59.2 months (range, 12.3–110 months) and the median age at diagnosis was 49.0 years (range, 25–76 years). Baseline and treatment characteristics are outlined in Table 1. Few patients, after NAC, with scant invasive tumor cells that were difficult to evaluate were grouped as not available (NA).
Table 1
| Variables | Total | Response | P value | |
|---|---|---|---|---|
| Yes [n (%)] | No [n (%)] | |||
| Age, years | 0.497 | |||
| <45 | 30 | 21 (23.6) | 9 (20.5) | |
| 45–65 | 82 | 52 (58.4) | 30 (68.2) | |
| ≥65 | 21 | 16 (18.0) | 5 (11.4) | |
| Clinical T status | 0.671 | |||
| 2 | 5 | 4 (4.5) | 1 (2.3) | |
| 2 | 71 | 50 (56.2) | 21 (47.7) | |
| 3 | 34 | 20 (22.5) | 14 (31.8) | |
| 4 | 23 | 15 (16.9) | 8 (18.2) | |
| Clinical positive LN number | 0.794 | |||
| 0 | 41 | 28 (31.5) | 13 (29.5) | |
| N1 | 78 | 53 (59.6) | 25 (56.8) | |
| N2 | 12 | 7 (7.9) | 5 (11.4) | |
| N3 | 2 | 1 (1.1) | 1 (2.3) | |
| Clinical stage | 0.242 | |||
| II | 76 | 54 (60.7) | 22 (50.0) | |
| III | 57 | 35 (39.3) | 22 (50.0) | |
| Clinical grade | 0.568 | |||
| Well | 4 | 2 (2.2) | 2 (4.5) | |
| Moderately | 90 | 59 (66.3) | 31 (70.5) | |
| Poorly | 33 | 24 (27.0) | 9 (20.5) | |
| NA | 6 | 4 (4.5) | 2 (4.5) | |
| Breast cancer subtypes | 0.119 | |||
| Luminal A (G1/2) | 46 | 26 (29.2) | 20 (45.5) | |
| Luminal B (G3) | 15 | 11 (12.4) | 4 (9.1) | |
| HER2 (ER-/PR-) | 19 | 16 (18.0) | 3 (6.8) | |
| Luminal-HER2 | 33 | 25 (28.1) | 8 (18.2) | |
| Triple-negative | 18 | 10 (11.2) | 8 (18.2) | |
| NA | 2 | 1 (1.1) | 1 (2.3) | |
| Chemotherapy | 0.001 | |||
| Neoadjuvant only | 95 | 72 (75.8) | 23 (24.2) | |
| Neoadjuvant + adjuvant | 38 | 17 (44.7) | 21 (55.3) | |
| Regimens of NAC | 0.289 | |||
| Anthracycline based | 48 | 30 (33.7) | 18 (40.9) | |
| Taxane based | 27 | 21 (23.6) | 6 (13.6) | |
| Anthracycline + taxane | 51 | 35 (39.3) | 16 (36.4) | |
| Others | 7 | 3 (3.4) | 4 (9.1) | |
| Hormone therapy | 0.809 | |||
| No | 35 | 24 (68.6) | 11 (31.4) | |
| Yes | 98 | 65 (66.3) | 33 (33.7) | |
| Trastuzumab usage | 0.013 | |||
| No | 83 | 48 (57.8) | 35 (42.2) | |
| Yes | 50 | 41 (82.0) | 9 (18.0) | |
| Surgical method | 0.056 | |||
| BCS | 64 | 52 (58.4) | 12 (27.3) | |
| Mastectomy | 69 | 37 (41.6) | 32 (72.7) | |
| Pathologic T status | <0.001 | |||
| 0 | 18 | 18 (20.2) | 0 (0.0) | |
| DCIS | 7 | 7 (7.9) | 0 (0.0) | |
| 1A | 22 | 21 (23.6) | 1 (2.3) | |
| 1B | 9 | 9 (10.1) | 0 (0.0) | |
| 1C | 22 | 20 (22.5) | 2 (4.5) | |
| 2 | 28 | 10 (11.2) | 18 (40.9) | |
| 3 | 17 | 3 (3.4) | 14 (31.8) | |
| 4 | 10 | 1 (1.1) | 9 (20.5) | |
| Pathologic positive LN number | <0.001 | |||
| 0 | 62 | 47 (52.8) | 15 (34.1) | |
| 1–3 | 42 | 33 (37.1) | 9 (20.5) | |
| 4–9 | 22 | 9 (10.1) | 13 (29.5) | |
| ≥10 | 7 | 0 (0.0) | 7 (15.9) | |
| Pathologic grade | <0.001 | |||
| No residual tumor | 18 | 18 (20.2) | 0 (0.0) | |
| DCIS | 7 | 7 (7.9) | 0 (0.0) | |
| Well | 14 | 6 (6.7) | 8 (18.2) | |
| Moderately | 56 | 36 (40.4) | 20 (45.5) | |
| Poorly | 33 | 17 (19.1) | 16 (36.4) | |
| Microinvasion only | 5 | 5 (5.6) | 0 (0.0) | |
| Downstaging | 86 | 74 (83.1) | 12 (27.3) | <0.001 |
| LVI | 78 | 40 (44.9) | 38 (90.5) | <0.001 |
| ENE | 41 | 17 (19.1) | 24 (55.8) | <0.001 |
| Surgical margin ≤1 mm | 32 | 12 (13.5) | 20 (45.5) | <0.001 |
| LR (local recurrence) | 12 | 5 (5.6) | 7 (15.9) | 0.001 |
| LR (axilla/SCF) | 12 | 7 (7.9) | 5 (11.4) | 0.036 |
| LR (both) | 4 | 0 (0.0) | 4 (9.1) | 0.011 |
| DM (bone) | 25 | 11 (12.4) | 14 (31.8) | 0.007 |
| DM (lung) | 14 | 8 (9.0) | 6 (13.6) | 0.549 |
| DM (liver) | 12 | 6 (6.7) | 6 (13.6) | 0.21 |
| DM (brain) | 7 | 3 (3.4) | 4 (9.1) | 0.219 |
| DM (other) | 6 | 2 (2.2) | 4 (9.1) | 0.093 |
| Death | 26 | 12 (13.5) | 14 (31.8) | 0.012 |
LN, lymph node; NA, not available; ER, estrogen receptor; PgR, progesterone receptor; HER-2, human epidermal growth factor receptor 2; NAC, neoadjuvant chemotherapy; BCS, breast conserving surgery; DCIS, ductal carcinoma in situ; LVI, lymphovascular invasion; ENE, extranodal extension; LR, locoregional recurrence; DM, distant metastasis; P value by Chi-square test or Fisher’s exact test when appropriated.
Various chemotherapy regimens were utilized, the most common of which were EC and/or T (epirubicin, cyclophosphamide, docetaxel), and FEC (5-fluoruracil, epirubicin, cyclophosph-amide). Fifty patients (37.6%) also received trastuzumab (Herceptin). Of these, 44 patients received it pre-operatively while six patients were treated in the adjuvant setting. One patient had neoadjuvant hormonal therapy with an aromatase inhibitor.
Surgery included an axillary lymph node dissection in 117 patients (88.0%), with the median number of lymph nodes removed being 13 nodes (range, 2–36 nodes). The remaining patients merely had sentinel lymph node biopsies.
All patients received adjuvant radiation therapy, which was delivered using conventional fields in 38 patients (28.6%), 3D conformal therapy in 34 patients (25.6%), intensity modulated radiation therapy (IMRT) in 30 patients (22.6%), and volume modulated arc therapy (VMAT) in 31 patients (23.3%). The median dose was 5000 cGy. A total of 95 of the 133 patients (71.4%) had irradiation to regional lymphatic areas. Seventy-seven patients (57.9%) received tumor bed boosts, with a median boost dose of 1,000 cGy. Only one patient had incomplete radiotherapy owing to rapidly progressing disease and her treatment was then replaced with palliative chemotherapy.
After NAC, 89 patients (66.9%) were responders and 44 patients (33.1%) were non-responders. Of the responders, 19 women, including 15 with ypT0N0 and four with ypTisN0, had a CR to treatment (14.3%), and 70 women had a PR (52.6%). Fourteen patients (18.4%) in clinical stage II had pCR while only five patients (8.8%) in clinical stage III had pCR. All of the patients who had pCR had no locoregional recurrence. Furthermore, of the 62 patients who were pathologically node-negative, 32 patients were observed to be clinically node-positive on the pretreatment evaluation.
The addition of trastuzumab to NAC significantly improved the pCR rate in patients with HER2/neu overexpression. Of the 44 patients with HER2/neu-overexpression that received at least one cycle of trastuzumab in the neoadjuvant setting, 12 achieved pCR (27.3%) and 38 were responders (pCR + PR, 86.4%), while in the group of eight patients with overexpression of HER2/neu, treated with NAC without trastuzumab, only one (Luminal-HER2 subtype) achieved pCR (12.5%) and three were responders (37.5%).
In total, 37 patients had distant metastases (27.8%) and 22 patients had more than one site metastasis. The most common sites were bone, lung, liver, and brain. Twenty-one patients with locoregional recurrence developed distant metastases synchronously or metachronously.
Between the responders and non-responders, OS was 94.5 and 78.2 months (95% CI, 89.2–99.8 and 67.0–89.4, P=0.007), and RFS was 85.5 and 59.9 months (95% CI, 78.3–92.6 and 47.3–72.4, P<0.001), respectively (Figure 3A,B). The time to locoregional recurrence was 56.7 and 48.3 months (responders vs. non-responders, P=0.033). Table 2 presents the multivariate Cox regression analyses of OS and RFS for the entire cohort. The Cox models confirmed the prognostic value of clinically HER2/neu-positive (HR =0.234; 95% CI, 0.074–0.737, P=0.0013), whereas pathologically node-positive patients were associated with poorer OS (HR =7.126; 95% CI, 1.748–29.055, P=0.006). As for RFS, responders (pCR/PR) showed significantly better results (P=0.033). However, pathologically node-positive patients displayed a trend in recurrence (P=0.057). For the non-responder group, multivariate Cox regression analysis of OS was performed and no significant prognostic factors were observed.
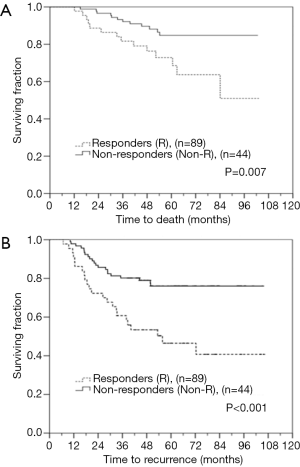
Table 2
| Survival | Multiple analysis | ||
|---|---|---|---|
| HR | 95% CI | P value | |
| Overall survival | |||
| LVI (+) | 1.825 | 0.616–5.407 | 0.278 |
| ENE (+) | 1.092 | 0.424–2.814 | 0.855 |
| Surgical margin ≤1 mm | 1.160 | 0.393–3.428 | 0.788 |
| Responder (pCR/PR) | 0.608 | 0.198–1.873 | 0.386 |
| Pathological N (+) | 7.126 | 1.748–29.055 | 0.006 |
| Poorly differentiation | 2.397 | 0.883–6.506 | 0.086 |
| cER (+) | 0.673 | 0.188–2.408 | 0.542 |
| cPR (+) | 0.417 | 0.107–1.621 | 0.206 |
| cHER2 (+) | 0.234 | 0.074–0.737 | 0.013 |
| Relapse-free survival | |||
| LVI (+) | 1.641 | 0.674–3.996 | 0.257 |
| ENE (+) | 1.038 | 0.399–2.701 | 0.939 |
| Surgical margin ≤1 mm | 0.884 | 0.363–1.986 | 0.765 |
| Responder (pCR/PR) | 0.406 | 0.177–0.93 | 0.033 |
| Pathological N (+) | 2.489 | 0.975–6.358 | 0.057 |
| Poorly differentiation | 1.625 | 0.77–3.431 | 0.203 |
| cER (+) | 0.667 | 0.271–1.646 | 0.38 |
| cPR (+) | 0.784 | 0.323–1.904 | 0.591 |
| cHER2 (+) | 0.845 | 0.413–1.729 | 0.644 |
LVI, lymphovascular invasion; ENE, extranodal extension; ER, estrogen receptor; HR, hazard ratio; PR, partial response; pCR, pathological complete response; HER2, human epidermal growth factor receptor 2.
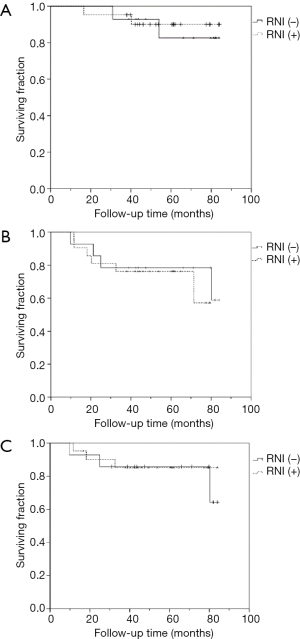
In the subgroup analysis, in order to evaluate the effects of breast + RNI after BCS or chest wall + RNI after mastectomy in patients who presented with cN(+) but converted to ypN0 following NAC, we further investigated the role of adjuvant RNI in cT1-3N1 diseases. In total 29 patients with cT1-3N1 who achieved ypN0 after NAC were selected. Of these 29 patients, 21 patients who underwent BCS or mastectomy and received comprehensive adjuvant radiotherapy (breast + RNI or chest wall + RNI) were in the comprehensive RNI group, while eight patients who had BCS and received adjuvant breast-only irradiation (without RNI) were in the non-RNI group. Moreover, from our database we also collected 6 additional patients with cT1-3N1 disease who became ypN0 after NAC and mastectomy but received no adjuvant radiotherapy. Consequently, there were 14 patients in the non-RNI group. Kaplan-Meier survival curves for comprehensive RNI vs. non-RNI were generated for OS {78.5 vs. 77.1 months [RNI(+) vs. RNI(−)], 95% CI, 71.1–85.9 and 68.3–86.0, P=0.750}, RFS {63.5 vs. 69.2 months [RNI(+) vs. RNI(−)], 95% CI, 52.4–74.6 and 55.3–83.3, P=0.589}, and locoregional relapse-free survival (LRRFS) {74.7 vs. 73.7 months [RNI(+) vs. RNI(−)], 95% CI, 64.9–84.5 and 61.5–85.9, P=0.855; Figure 4A,B,C}.
Discussion
With the aim of identifying patients for whom NAC is likely to yield a beneficial effect, we analyzed the differences between responders (either partial or CR) and non-responders in distributions of clinical and pathological features, while many previous studies focused on achieving pCR as a primary endpoint (11-13). Our findings revealed that response (responders vs. non-responders) to NAC in clinical stage II and III breast cancer had a prognostic impact on RFS and OS (P<0.001 and P=0.007, respectively). This significant difference may suggest the use of the responder vs. non-responder classification for tumor evaluation in clinical practice.
The NOAH trial reported that one year of initiating with trastuzumab as a neoadjuvant almost doubled pCR rates, and the total pCR for HER2/neu-positive individuals treated trastuzumab was 38% (7). In our study, the addition of trastuzumab to NAC for HER2/neu-positive patients showed a pCR rate of 27.3%. This outcome may be due to the fact that the HER2/neu-positive subtype is likely to have the highest activation of the EGFR-HER2/neu pathway and to benefit the most from the trastuzumab. Furthermore, none of the patients with pCR (n=19) in our study had locoregional recurrence after 5 years of follow-up. However, one of the pCR patients, who had initial cT2N1M0 poorly differentiated breast invasive ductal carcinoma, got brain and bone metastases after 20 months of follow-up and expired 20 months later.
A pooled analysis of two large prospective randomized-controlled trials of NAC: NSABP B-27 and B-18, which both prohibited patients from having adjuvant radiotherapy, concluded that, apart from initial tumor characteristics before NAC, pathological responses in the breast or lymph node also have major impacts on rates of locoregional recurrence (3,14,15). Based on our findings in the multivariate analysis of the entire cohort, it appears that patients who are clinically HER2/neu-positive may benefit in OS by receiving NAC, surgery, and adjuvant RT as their definitive treatment and those who are responders may also have significantly better RFS. Thus, the dichotomic method of response (responders vs. non-responders) after NAC in our study may be appropriate for response evaluation of NAC patients as well as providing a simple and a useful clinical prognostic marker for RFS. Similarly, a Korean study aimed to identify a simple and reproducible tool for response evaluation of NAC patients, and found the AJCC response criteria to be a useful clinical prognostic marker for RFS. Moreover, it is noteworthy that in our study, for patients who were pathologically node-positive, we observed a clear trend of poor RFS and a significantly poor OS, even after adjuvant radiotherapy (16).
In the era of neoadjuvant treatment, the non-responder category has gained little attention in the literature. However, in the Cox regression analysis of OS and RFS for the non-responder group, no statistically significant factors were noted. The unfavorable response of non-responders to NAC may be related to tumor heterogeneity and intrinsic tumor characteristics, and non-responders merit further investigation by other up-to-date methods, such as gene expression profiling (17,18).
In addition, we also found that a greater number of patients with a close surgical margin (≤1 mm), LVI, and ENE were associated with being non-responders (all P<0.001). This may imply that the extent of the invasive tumor in the non-responders is poorly delineated and clear surgical margins may be not easily obtained after NAC. The SSO-ASTRO Consensus Guideline on Margins for Breast-Conserving Surgery with Whole Breast Irradiation in Stage I and II Invasive Breast Cancer excluded patients treated with NAC owing to the “buckshot” pattern of response to NAC. The guideline also raised concerns that margins of no ink on tumors may be associated with a significant residual tumor burden after NAC (19,20). However, in our study, these factors [close surgical margin (≤1 mm), LVI, and ENE] did not turn out to be poor prognostic factors of OS for the non-responder group. This might be due to either the short follow-up duration or the effects of adjuvant therapy.
Moreover, in our subgroup analysis, there was no statistical difference between clinically HER2/neu-positive and clinically HER2/neu-negative patients in locoregional recurrent rates (21.2% vs. 21.5%, P=0.960), whereas we observed a clear trend whereby there were more clinically HER2/neu-negative patients suffering from distant metastasis (19.2% vs. 34.2%, P=0.063). This further establishes that the HER2/neu overexpression subtype, with the highest activation of the EGFR-HER2/neu pathway, benefits the most from the trastuzumab, and that the heterogeneous tumor characteristics of breast cancer resulted in the unfavorable distant metastatic rate in the clinically HER2/neu-negative group (17).
Another issue for NAC in invasive breast cancer is the role of adjuvant RNI in patients with pathologically node-negative status. We learned from the NSABP B-27 and B-18 trials that NAC led to pN0 disease in around 20–40% of patients with early stage breast cancer. The regional nodal failure rate was <5% after mastectomy without adjuvant radiotherapy (2,21). Besides, comprehensive adjuvant radiotherapy was found to enhance locoregional control and survival in patients with clinical stage III–IV disease after NAC. Patients with cT3-4, cN2-3, stage III disease are recommended to undergo adjuvant radiotherapy according to the NCCN guidelines for invasive breast cancer, version 1.2017 (8,9,22-24). However, whether adjuvant RNI can provide survival benefits for patients with early stage breast cancer who were clinically node-positive but converted to pathologically node-negative status after NAC is still controversial. Thus, we tried to investigate differences in LRRFS, RFS, and OS between comprehensive and breast-only adjuvant radiotherapy in clinically node-positive early stage breast cancer. In the subgroup analysis using the Kaplan-Meier survival curves, there were no significant differences in OS, RFS, or LRRFS between the comprehensive and breast-only adjuvant radiotherapy groups (Figure 4). It seems that the effect of breast-only adjuvant radiotherapy might possibly be equivalent to effects found for the comprehensive group. Yet, owing to the limited sample size and relative short follow-up duration, further studies, such as the ongoing NSABP B-51 prospective trial, are required (25).
Our study has some limitations. First, the study was retrospective and was based on data from different clinical practices. Therefore, selection bias cannot be excluded. Second, the sample sizes of each subgroup were limited. The subgroup analysis for the role of adjuvant RNI should be validated in a larger population. Finally, although our study had a median follow-up time of 59.2 months, a longer follow-up would be beneficial to explore the subgroup analysis of adjuvant RNI.
Conclusions
Clinically, HER2/neu-positive status was a strong predictor of response when we utilized trastuzumab for patients who were clinically HER2/neu-positive in our study. HER2/neu-enriched patients also had fewer distant metastases than clinically HER2/neu-negative patients after neoadjuvant treatment. The dichotomic method of response (responders vs. non-responders) after NAC in our study may be a useful clinical prognostic marker for relapsed-free survival in stage II and III invasive ductal carcinoma of the breast. For the non-responder group, due to the heterogeneity and intrinsic characteristics of tumors, outcomes are dismal. Thus, this group should be the subject of further genetic profiling studies. Regarding adjuvant RNI, although our results reveal that the survival outcome of breast-only adjuvant radiotherapy may possibly be similar to that for the comprehensive group, ongoing prospective clinical trials like the NSABP B-51 trial may answer some of the unresolved issues.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was conducted according to the ethical and institutional rules concerning research on patients and was approved by the breast cancer multidiscipline group of Changhua Christian Hospital. Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Health Promotion Administration, Ministry of Health and Welfare (Taiwan). Cancer Registry Annual Report 2014;68-9.
- Garg AK, Buchholz TA. Influence of neoadjuvant chemotherapy on radiotherapy for breast cancer. Ann Surg Oncol 2015;22:1434-40. [Crossref] [PubMed]
- Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project protocols B-18 and B-27. J Clin Oncol 2008;26:778-85. [Crossref] [PubMed]
- Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 2001;(30):96-102.
- Chang HR. Trastuzumab-based neoadjuvant therapy in patients with HER2-positive breast cancer. Cancer 2010;116:2856-67. [Crossref] [PubMed]
- Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol 2012;2:62. [Crossref] [PubMed]
- Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 2010;375:377-84. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: Breast Cancer, v1.2017.
- Buchholz TA, Lehman CD, Harris JR, et al. Statement of the science concerning locoregional treatments after preoperative chemotherapy for breast cancer: a National Cancer Institute conference. J Clin Oncol 2008;26:791-7. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Marchio C, Sapino A. The pathologic complete response open question in primary therapy. J Natl Cancer Inst Monogr 2011;2011:86-90.
- Penault-Llorca F, Abrial C, Raoelfils I, et al. Comparison of the prognostic significance of Chevallier and Sataloff's pathologic classifications after neoadjuvant chemotherapy of operable breast cancer. Hum Pathol 2008;39:1221-8. [Crossref] [PubMed]
- Untch M, Fasching PA, Konecny GE, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol 2011;29:3351-7. [Crossref] [PubMed]
- Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: Preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2003;21:4165-74. [Crossref] [PubMed]
- Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowell Project B-18. J Clin Oncol 1997;15:2483-93. [Crossref] [PubMed]
- Yang Y, Im SA, Keam B, et al. Prognostic impact of AJCC response criteria for neoadjuvant chemotherapy in stage II/III breast cancer patients: breast cancer subtype analyses. BMC Cancer 2016;16:515. [Crossref] [PubMed]
- Balmativola D, Marchiò C, Maule M, et al. Pathological non-response to chemotherapy in a neoadjuvant setting of breast cancer: an inter-institutional study. Breast Cancer Res Treat 2014;148:511-23. [Crossref] [PubMed]
- Oakman C, Santarpia L, Di Leo A. Breast cancer assessment tools and optimizing adjuvant therapy. Nat Rev Clin Oncol 2010;7:725-32. [Crossref] [PubMed]
- Buchholz TA, Somerfield MR, Griggs JJ, et al. Margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer: American Society of Clinical Oncology endorsement of the Society of Surgical Oncology/American Society for Radiation Oncology consensus guideline. J Clin Oncol 2014;32:1502-6. [Crossref] [PubMed]
- Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol 2014;32:1507-15. [Crossref] [PubMed]
- Hamy-Petit AS, Belin L, Bonsang-Kitzis H, et al. Pathological complete response and prognosis after neoadjuvant chemotherapy for HER2-positive breast cancers before and after trastuzumab era: results from a real-life cohort. Br J Cancer 2016;114:44-52. [Crossref] [PubMed]
- Akyurek S, Yavas G. Role of postmastectomy radiation therapyafter neoadjuvant chemotherapy in locally advanced breast cancer. Exp Oncol 2013;35:267-71. [PubMed]
- Huang EH, Tucker SL, Strom EA, et al. Predictors of locoregional recurrence in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy, mastectomy, and radiotherapy. Int J Radiat Oncol Biol Phys 2005;62:351-7. [Crossref] [PubMed]
- Liu J, Mao K, Jiang S, et al. The role of postmastectomy radiotherapy in clinically node-positive, stage II-III breast cancer patients with pathological negative nodes after neoadjuvant chemotherapy: an analysis from the NCDB. Oncotarget 2016;7:24848-59. [PubMed]
- A randomized phase III clinical trial evaluation post-mastectomy chestwall and regional nodal XRT and post-lumpectomy regional nodal XRT in patients with positive axillary nodes before neoadjuvant chemotherapy who convert to pathologically negative axillary nodes after neoadjuvant chemotherapy. Available online: https://www.owensborohealth.org/patient-visitor/clinical-trials/details/?id=35
Cite this article as: Wang SH, Pi CP, Chang TH, Liu MT, Huang CC, Hung LC, Chou TW, Chang YJ. The impacts of chemotherapeutic response for clinical stage II and III breast cancer patients after neoadjuvant chemotherapy. Ther Radiol Oncol 2018;2:13.


