
Cutaneous squamous cell carcinoma of the head and neck with parotid gland metastasis mimicking mucoepidermoid carcinoma: a potential diagnostic pitfall
Introduction
Parotid tumors affected 1:100,000 people with the highest incidence found in Northern Queensland, Australia (1). About 75% of the parotid masses are benign and among them, pleomorphic adenoma accounted for the majority. On the other hand, primary parotid cancer is rather uncommon, representing only 2–3% of all head and neck malignancy (2,3). The most frequently encountered primary malignancy is mucoepidermoid carcinoma (MEC). Parotid gland metastasis is rare on the contrary. The most common site of origin is the skin of the cutaneous head and neck squamous cell carcinoma (cHNSCC), and it develops in 1–3% of patients (4). In the report, we present an unusual case of the parotid gland metastasis from cHNSCC, and the patient did not have the apparent skin lesion on the surface, radiological findings mimicked that of primary MEC. Special attention should be paid to the potential cause of diagnostic pitfalls.
Case report
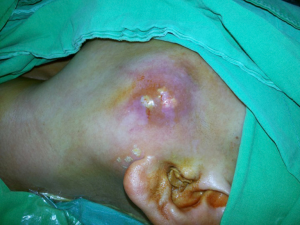
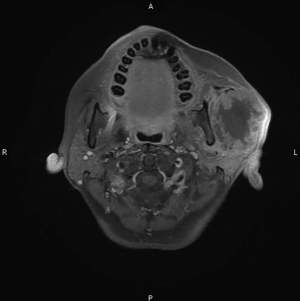
This is a 56-year-old female who presented with a mass at the left pre-auricular region that slowly enlarged during the previous one year. At first, there was only a bulging mass without apparent skin lesion or skin defect. Because it was asymptomatic, the patient paid no attention to it. However, progressive left-sided facial palsy and numbness developed in 1 month before this admission. A firm mass of the left parotid gland was 4 cm in diameter on palpation (Figure 1). The severity of facial palsy was grade VI according to House-Brackmann Facial Nerve Grading System (4-6). Magnetic resonance images revealed a large lobulated mass measuring 51 mm × 55 mm × 56 mm within the left parotid gland, which invaded the ipsilateral masseter muscle and compressed the left lateral pterygoid muscle (Figure 2). Our experienced diagnostic radiologist made a provisional diagnosis of MEC. Other image studies showed no evidence of distant metastasis. The patient received left total parotidectomy with ipsilateral supraomohyoid neck dissection.
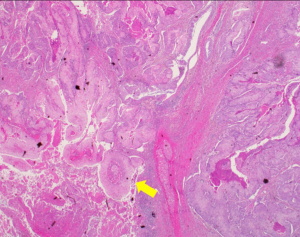
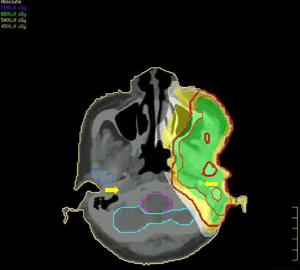
The surgical specimen submitted for routine microscopic examination. Grossly, the tumor was well encapsulated and measured 62 mm × 50 mm × 40 mm in size. Histological features showed tumor cells with prominent nucleoli arranged in solid nest pattern accompanied by focal keratinization (Figure 3). The immunohistochemical analysis for mucin was negative which excluded the diagnosis of MEC. The final diagnosis revised to cHNSCC with parotid lymph node metastasis according to the pathologic findings. The cancer staging was pT0N3M1, stage IV based on 7th AJCC staging system: the T0 was assigned T category due to no primary found; the N3 was assigned N category due to intraparotid lymph node involvement with more than 6 cm in greatest dimension; and M1 was assigned M category due to parotid gland metastasis. According to the O’Brien staging system is P3N0. Then the patient received adjuvant radiotherapy five fractions per week with 66 Gy to the tumor bed and high-risk area including ipsilateral level II–III and involved facial nerve root retrogradely; 54 Gy to the ipsilateral level IV (Figure 4). The patient received concurrent chemotherapy with weekly cisplatin and completed treatment without any interruption or any intolerable acute toxicity.
Discussion
There are at least 20 subtypes of histology of primary major salivary gland tumor and around 5% of primary major salivary gland tumors are squamous cell carcinoma (7). The primary SCC of the parotid gland is a diagnosis by exclusion. According to the Tanvetyanon et al. stated the pathological finding of the primary SCC when arises in the salivary gland frequently replaces the whole glands and the small remnants of metaplastic ducts or degenerating acini within desmoplastic fibrotic background are always identified (7). Thus, the final diagnosis of this patient is regarded as metastatic SCC of the head and neck skin. In generally, cHNSCC constitutes about 20–25% of all skin cancer and is known to have a higher incidence of local-regional involvement (5). A systematic analysis, that carries out recently suggested that the risk of nodal metastasis for cHNSCC is about 5% and among them, the parotid gland nodes are most frequently involved (60–70%) (2). Risk factors for nodal involvement in cHNSCC include tumor size larger than 2cm, thickness over than 4mm, presence of lymphovascular invasion (LVSI), perineural invasion (PNI) or an immunocompromising condition.
PNI are identified in 3.7–6% of cHNSCC patients with the trigeminal nerve (CN V) and the facial nerve (CN VII) being the most commonly involved (8,9). The typical symptoms for patients with PNI are numbness, pain, or paresthesia (9). The incidence of partial or complete facial nerve sacrifice was varies from 5% to 43% in cHNSCC, who underwent complete or partial parotidectomy. Patients with malignant facial nerve palsy or facial nerve invasion often have a poor prognosis (10). In our reported case, the patient had the clinical symptoms of malignant left-sided facial nerve palsy, which could be explained by our pathological finding of PNI.
Two different staging systems has proposed for cHNSCC: the American Joint Committee on Cancer (AJCC) TNM and the O’Brien staging systems. The AJCC TNM system has criticized as an inadequate staging approach for skin cancer because of its inability to discriminate between the number, size, and location of nodes. In TNM systems, parotid and cervical nodes had categorized into the same regional group. By contrast, O’Brien et al. suggested a staging system that separates node involvement into P (parotid node) and N (cervical node) stages respectively (Table 1) (10). The patient presented cutaneous hand and neck cancer with parotid gland involvement and parotid gland metastasis, thus, the O’Brien staging system might be more suitable for this patient.
Table 1
| O’Brien clinical staging |
| Parotid |
| P0 (no clinical disease in the parotid) |
| P1 (metastatic node ≤3 cm in diameter) |
| P2 (metastatic node >3 cm but ≤6 cm in diameter, or multiple nodes) |
| P3 (metastatic node >6 cm in diameter or disease involving 7th nerve or skull base) |
| Neck |
| N0 (no clinical disease) |
| N1 (single ipsilateral neck node ≤3 cm in diameter) |
| N2 (single node >3 cm in diameter or multiple nodes or contralateral nodes) |
| AJCC-7 tumor staging system for cutaneous squamous cell carcinoma of the head and neck TNM staging |
| Primary Tumor (criteria) |
| TX (primary tumor cannot be assessed) |
| T0 (no evidence of primary tumor) |
| Tis (carcinoma in situ) |
| T1 (tumor ≤2 cm in greatest dimension) |
| T2 (tumor >2 cm in greatest dimension or any size with ≥2 high-risk features) |
| T3 (tumor with invasion of maxilla, mandible, orbit, or temporal bone) |
| T4 (tumor with invasion of skeleton (axial or appendicular) or perineural invasion of skull base) |
| Regional lymph node (criteria) |
| NX (regional lymph nodes cannot be assessed) |
| N0 (no regional lymph node metastasis) |
| N1 (metastasis in a single ipsilateral lymph node, ≤3 cm in greatest dimension) |
| N2a (metastasis in a single ipsilateral node, >3 cm but ≤6 cm in greatest dimension) |
| N2b (metastasis in multiple ipsilateral nodes, ≤6 cm in greatest dimension) |
| N2c (metastasis in bilateral or contralateral lymph nodes, ≤6 cm in greatest dimension) |
| N3 (metastasis in a lymph node, more than 6 cm in greatest dimension) |
| M0 (no distant metastasis) |
| M1 (distant metastasis) |
Recent publications recommended medically operable patients to receive surgery followed by postoperative RT (4-6). A retrospective study by Veness et al. demonstrated that surgery and adjuvant radiotherapy provide a lower loco-regional recurrence and better 5-year disease-free survival in cHNSCC patients with metastasis to the lymph nodes (5). Most recent studies suggested a regimen of adjuvant radiotherapy of 60 Gy to nodal region that has already been dissected and 50 Gy to the at-risk nodal area which has not been dissected (5).
Therefore, under the highly conformal radiotherapy, the identification and delineation of the clinical target volumes (CTVs) for regions at risk is the most importance. Eisbruch et al. reported guidelines for CTV delineation in the treatment of cHNSCC with PNI based on their systematic analysis of patterns of disease progression and treatment failures. Auriculo-temporal nerve and the greater superficial petrosal nerve (GSPN) are the communicating branches between the terminal branches of CN V and CN VII. The CTV for conformal RT should include CN V and VII due to high risk for primary tumor involvement (8).
Only few sparse studies have compared the prognosis for patients who received adjuvant chemoradiation to patients who received adjuvant radiation for cHNSCC. Tanvetyanon et al. reported that adjuvant concurrent chemoradiation was associated with a reduced regional progression and an improved recurrence-free survival in advanced, high-risk patients (7). Hinerman et al. recommended that chemoradiation should be used in selected patients with positive margins and/or extensive nodal disease due to poor prognosis (4). In accordance with the results of the studies mentioned above, this patient received adjuvant chemoradiotherapy for the treatment.
Patients with operable cHNSCC best treaties by surgery and adjuvant radiotherapy. If the patient was diagnosed to have cHNSCC with parotid metastasis and PNI, the CTV for adjuvant radiotherapy should include the entire involved nerve. For those patients with high risk of relapse, the more intensive treatment such as the concurrent use of chemotherapy to adjuvant radiotherapy should recommend.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (Available at https://tro.amegroups.com/article/view/10.21037/tro-02-9/coif). HLC serves as an unpaid editorial board member of Therapeutic Radiology and Oncology from Apr 2020 to Mar 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Makki FM, Mendez AI, Taylor SM, et al. Prognostic factors for metastatic cutaneous squamous cell carcinoma of the parotid. J Otolaryngol Head Neck Surg 2013;42:14. [Crossref] [PubMed]
- Clark J, Wang S. Metastatic Cancer to the Parotid. Adv Otorhinolaryngol 2016;78:95-103. [Crossref] [PubMed]
- Maahs GS, Oppermann Pde O, Maahs LG, et al. Parotid gland tumors: a retrospective study of 154 patients. Braz J Otorhinolaryngol 2015;81:301-6. [Crossref] [PubMed]
- Hinerman RW, Indelicato DJ, Amdur RJ, et al. Cutaneous squamous cell carcinoma metastatic to parotid-area lymph nodes. Laryngoscope 2008;118:1989-96. [Crossref] [PubMed]
- Veness MJ, Morgan GJ, Palme CE, et al. Surgery and adjuvant radiotherapy in patients with cutaneous head and neck squamous cell carcinoma metastatic to lymph nodes: combined treatment should be considered best practice. Laryngoscope 2005;115:870-5. [Crossref] [PubMed]
- Audet N, Palme CE, Gullane PJ, et al. Cutaneous metastatic squamous cell carcinoma to the parotid gland: analysis and outcome. Head Neck 2004;26:727-32. [Crossref] [PubMed]
- Tanvetyanon T, Padhya T, McCaffrey J, et al. Postoperative concurrent chemotherapy and radiotherapy for high-risk cutaneous squamous cell carcinoma of the head and neck. Head Neck 2015;37:840-5. [Crossref] [PubMed]
- Gluck I, Ibrahim M, Popovtzer A, et al. Skin cancer of the head and neck with perineural invasion: defining the clinical target volumes based on the pattern of failure. Int J Radiat Oncol Biol Phys 2009;74:38-46. [Crossref] [PubMed]
- Sapir E, Tolpadi A, McHugh J, et al. Skin cancer of the head and neck with gross or microscopic perineural involvement: Patterns of failure. Radiother Oncol 2016;120:81-6. [Crossref] [PubMed]
- O'Brien CJ, McNeil EB, McMahon JD, et al. Significance of clinical stage, extent of surgery, and pathologic findings in metastatic cutaneous squamous carcinoma of the parotid gland. Head Neck 2002;24:417-22. [Crossref] [PubMed]
Cite this article as: Liu MY, Lo CH, Liao YS, Chao HL, Lin KT, Huang WY, Shen PC, Tao CC, Chen CM, Lin CS. Cutaneous squamous cell carcinoma of the head and neck with parotid gland metastasis mimicking mucoepidermoid carcinoma: a potential diagnostic pitfall. Ther Radiol Oncol 2018;2:9.


