
Chordoid glioma of the third ventricle—a case report and literature review
Introduction
Chordoid glioma (CG) is a low-grade brain tumor originating from the thalamus or anterior wall of the third ventricle. It was classified as grade II glioma according to the WHO since 2000 (1). Less than 100 cases have been reported to the public indicating its rarity so far (2). Zarghouni et al. reviewed previous cases reported which concluded that CG affected women more than men at a rate of 2:1 and the age of diagnosis were most likely between 30 and 60 years old (3). To be further, CG is a type of brain tumor which has its unique histologic appearance and specific location (4). Neuroradiologically, the tumor tends to be ovoid in shape and locates in the region of the hypothalamus/anterior third ventricle with uniform enhancement and intensity in magnetic resonance imaging (MRI) scans (5). Histologically, clusters and cords of oval-to-polygonal epithelioid tumor cells with abundant eosinophilic cytoplasm are seen as the hallmark of the CG (6). CG is most treated with maximal surgical removal in the literature. If gross total resection (GTR) was not possible due to the location and the size of the tumor, subtotal resection (STR) was often the choice of treatment. After STR, few were submitted to adjuvant radiotherapy. There’re controversies regarding the dosage and the technique of radiotherapy for this disease.
Here we present a case of CG over the third ventricle who received external beam radiotherapy after stereotactic brain biopsy. The roles of radiotherapy and other clinical presentations were discussed subsequently in more detail and literatures were reviewed as well.
Brief history
A 44-year-old man presented to our hospital with 2 weeks of severe headache, progressive unsteady gait and dizziness. Mild slurred speech was also found by the family for about a month. There was no sensory or motor function impairment, language function impairment, memory loss, arrhythmia, change in urination frequency, change in bowel habit, or change in visual fields. MRI showed an ovoid long T2 lesion with faint scattered contrast enhancement arising from right side of 3rd ventricular region, measuring 53 mm × 46 mm in diameter, majorly involving right thalamus, and causing obstructive hydrocephalus (Figure 1). Brain tumor was first suspected with increased intra-cranial pressure (IICP). The neurological symptoms of the patient mentioned above, including headache, unsteady gait and dizziness, recovered with controlling the IICP by steroids and glycerol. Only slurred speech continued after the IICP was controlled. The patient’s Karnofsky score was 90 and Eastern Cooperative Oncology Group (ECOG) performance score was 1 before any treatment started.
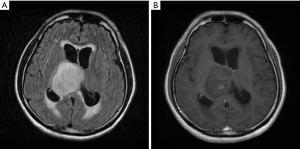
The patient then received a stereotactic brain biopsy. The diagnosis was CG. The tumor revealed epithelioid neoplastic cells with cord-like structures within a myxoid-rich stroma. The immunohistochemical (IHC) staining profiles showed positivity for GFAP, S-100 and CD34, negativity for EMA, with a Ki-67 index of 6.1% (Figure 2). The surgeon considered the tumor inoperable due to the location of the tumor. Local treatment by radiotherapy was suggested and accepted by the patient and family.

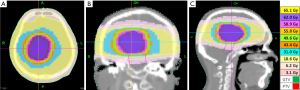
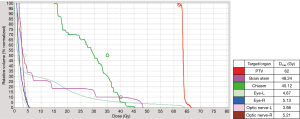
The patient was immobilized with a head rest, custom thermoplastic mask and underwent 3 mm sliced CT scan with contrast enhancement for simulation. The gross tumour volume (GTV) was contoured and a 3 mm margin was added to create planning target volume (PTV). The patient received radiotherapy using Helical TomoTherapy® (Accuray Incorporated, Sunnyvale, CA, USA). TomoTherapy® uses a fan-beam delivery system to deliver rotational intensity-modulated radiation therapy. The course of fractionated radiotherapy consisted of 62 Gy/31 fractions given 5 days per week of a period of 7 weeks. The brainstem was limited to less than 55 Gy and optic chiasm, lens, and optic nerves were also contoured with constrains to fulfill current standards for organ at risks. Ninety-eight percent of the PTV was covered by 62 Gy isodose curve line, and total GTV was covered 100% (Figure 3). The dose-volume histogram and dose to the region of interest (ROI) were illustrated in Figure 4. The patient received the radiotherapy uneventfully and the slurred speech recovered after the treatment. The acute side effect of the treatment was mild headache only. No other neurological sequelae developed during or after the treatment. The patient maintained his previous Karnofsky and ECOG scores, 90 and 1, respectively.
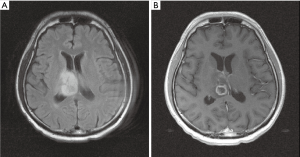
MRI which has been done one month after radiotherapy showed decreased tumor size (28.6 mm × 18.5 mm) as compared to prior MRI (53 mm × 46 mm). Focal cystic change with marginal contrast enhancement was noted within the tumor (Figure 5). However, tumor regrowth was noted 5 months later in follow-up MRI (Figure 6). The regrowth of the tumor was observed from the same location, which was within the GTV field. We considered it a residual tumor with progression. Several episodes of headache and unsteady gait have been noted due to IICP and controlled by medication during the follow-up period. The patient lost to follow-up 6 months after the treatment. The patient developed high fever with urosepsis and visited our emergency department 10 months after the radiotherapy. His condition deteriorated rapidly and expired due to multi-organ failure.

Discussion
Clinical presentation
To begin with, less than 100 cases of CG were reported in the literature with strikingly similar clinical features. The median age at diagnosis was 48 years (7). The female to male ratio was reported to be 2:1 (3). The median tumor diameter was about 3 cm, ranged from 1.5 to 7 cm. The clinical presentation was highly associated with the location of the tumor. Most part CG arose from the thalamus or the anterior wall of the third ventricle. Thalamus was believed to act as a relay between the subcortical area and the cerebral cortex. Thalamus possesses an important role for different functions of human body such as cognition, sensory function, motor and language function, memory, consciousness and the circadian rhythm. Tumors which originated from the inferior portion of the anterior third ventricle could bring about endocrine complaints which comprised diabetes insipidus (DI) and hypopituitarism (8). In the review article written by Ampie et al., the most common symptoms presented for CG were headache (40%), visual symptoms (35.8%), and mental status changes (27%) (7). The patients often experienced headache or other neurological symptoms over a long period of time prior to the diagnosis and its mean time from onset of symptoms to surgical management was 22.1 months (range, 0–20 years) (9).
In our case report, the patient reported to have intermittent headache for years and the clinical presentation included headache, dizziness and unsteady gait. After controlling IICP, only slurred speech was noted before his treatments. The patient has only slurred speech prior to the treatment after controlling the IICP.
Radiological findings
MRI was usually the better mean of imaging examination for tumor which arose from the third ventricle or thalamus. The differential diagnosis for solid tumors from this area included ependymoma, germinoma, brain metastasis and CG. Ependymoma which located in the supratentorial periventricular region generally appeared variably from strong solid enhancing masse to more heterogeneous mass with cystic component. Germinoma arose majorly from pineal region and also along the floor of the third ventricle, appearing with contrast enhancing solid mass. Brain metastasis should always be considered in adult brain mass whenever systemic malignancy was noted. CG usually presented as an ovoid solid mass known as hypo or isointense on T1-weighted MRI imaging with strong homogeneous contrast enhancement (10). Around 10% of the cases, CG might demonstrate heterogenous enhancement on T1 weighted MRI (11). On T2 weighted MRI, CG frequently appeared as hyperintense or isointense lesions (12). The findings of CT for CG typically appeared both circumscribed and hyperdense lesions that enhanced homogenously after contrast administration. However, the findings in the literature were inconsistent. Atypical CT features reported including intratumoral hemorrhage, heterogeneous contrast enhancement, and calcification. To conclude, our case indicated the relatively untypical appearance of CG in his diagnostic MRI as well as CT images. The tumor only showed faint scattered contrast enhancement in T1-weighted magnetic resonance (MR) image. In CT scan, the lesion was hypodense without homogeneous enhancement. Otherwise, the tumor was consistent with the findings in the literature. In T1-weighted MR image, the tumor revealed hypodense ovoid lesion. In T2-weighted MR image, it showed a hyperintense lesion.
Histological diagnosis
The characteristic of CG has its unique histologic and IHC features. Histologically, CG would resemble chordomas and chordoid meningiomas in its features of the clusters and cords of epithelioid cells within a mucinous vacuolated stroma (13). The IHC features included the positivity for GFAP, EMA, CD34, cytokeratin, S100 and vimentin. Lymphocyte infiltration was also commonly seen. The proliferative activity was generally low for CG. Mitoses were consistently low and no necrosis was ever reported (9,14-16). Desouza et al. have reviewed 50 cases of CG and indicated that the lymphocytic and plasma cell infiltrates were discussed in the ratio of 48 to 50 cases (96%). Likewise, the mucinous or myxoid stroma was discussed in the ratio of 49 to 50 cases. However, significant mitoses have only been found in 1 of the 45 cases so far. Ki-67 was recorded in 22 cases and ranged from 0.3% to 5% (17). Sanches et al. reported a unique case, a 59-year-old woman with a 2 cm CG, demonstrating GFAP and vimentin positivity, EMA negativity, and Ki-67 expression 20% (10). In our case, the tumor revealed epithelioid neoplastic cells with cord like structure in myxoid-rich stroma. The IHC stain showed positivity for GFAP, S-100 and CD34, and negativity for epithelial membrane antigen (EMA). Additionally, the Ki-67 index was 6.1% and it was slightly higher among other cases reviewed.
Treatments
CG was most generally treated by surgical resection. The surgical approaches included trans-lamina terminalis, transcallosal resection, and transcortical resection. Less than half of the patients (44%) were amenable for GTR due to the location of the tumor. Approximately, 45% of the cases received STR and 10% of them only received biopsy. In addition, complications after GTR, including hypothalamic dysfunction and transient DI, were in the percentage of 22.2 and 16 respectively. GTR remained the most prognostic factor for CG. No tumor recurrences were reported in the GTR group where 1-, 3-, and 5-year progression free survival (PFS) for STR was 85.2%, 71.0%, and 35.5% respectively (P=0.0276) in the literature (7). Moreover, some studies comprised of radiotherapy partly from the treatment for CG. The radiotherapy techniques included stereotactic radiosurgery (SRS), conventional external beam radiotherapy (EBRT), and intra-tumor radiotherapy (ITRT) using Iridium-192 (Ir-192) seed implantation. Above all, SRS was the majority reported in the review of the literatures. Reifenberger et al. reported the first case ever who received gamma-knife radiosurgery (GKRS) after STR for CG in a 56-year-old woman. The patient has been alive for 3.5 years (15). Nakajima et al. proposed that a 49-year-old woman received STR followed by SRS and its report has showed no regrowth of tumor within 2 years. The SRS was delivered by GKRS with marginal dose of 15–18 Gy (18). Chung et al. reported a patient that the recurrence at 8 months following STR and GKRS (marginal dose 20 Gy) required subsequent resection for recurrence (19). Kobayashi et al. indicated that two cases receiving only biopsy followed by GKRS with marginal dose 10.5–11 Gy, and were clear for diseases 70 and 66 months after the radiosurgery, respectively. Another case published from the same group was that a 61-year-old woman underwent STR and GKRS (marginal dose 12 Gy) has been clear for disease for 12 months before incidental death from an accident (20). In addition, the ITRT by using radioactive seed insertion was also reported in the literature. Kurian et al. brought out two cases of CG in their thirties both treated by ITRT with Ir-192. In both cases, one who received STR and Ir-192 implantation has been alive for at least 15 months in the follow-up. However, one who received biopsy following by Ir-192 recurred after 9 months died shortly after the repeat surgery for the recurrent tumor. The detail of the prescription for the Ir-192 treatment was not presented in the original article (21).
Nevertheless, the combination of SRS and conventional EBRT was reported in one case study by Tonami et al. They mentioned that a 42-year-old woman with a 1-year history of amenorrhea and memory impairment was given a diagnosis of CG which treated with STR. A total of 52 Gy of EBRT in conjunction with a 20 Gy delivered by SRS was given after the surgery. The patient was clear for tumor recurrence after 9 months follow-up (22).
Moreover, the use of EBRT along with or without STR was sparse in the literature. Brat et al. reported that two cases who received STR followed by EBRT. One 59-year-old man who received STR and EBRT developed recurrence and expired after 3 years. A 31-year-old female received STR and adjuvant EBRT has been alive for 4 years by follow-up (23). Hanbali et al. reported that a 57-year-old man with the history of hypertension, diabetes mellitus and an old myocardial infarction presented with a 1.5 cm oval lesion centered in the lamina terminals region. He received STR followed by EBRT in the University of Texas, MD Anderson Cancer Center. The dosage of EBRT was 54 Gy in 30 fractions. He developed headache, short-term memory loss, and worsening confusion and disorientation 7 months after the EBRT. Image studies revealed tumor progression (2 cm in diameter) which was removed totally. The patient died of a massive myocardial infarction 2 months after the salvage surgery (24). To date, Kobayashi et al. reported the best result for those who received STR or biopsy alone followed by radiotherapy, since both cases from their study survived for more than 5 years (20). The cases reported in the literatures that include radiotherapy in the treatment were summarized in Table 1 (15,18-24).
Table 1
| Case No. | First author (reference) | Year of publication | Age/sex | Initial procedure | RT technique | RT dose | Recurrence or progression | Retreatment | Status | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Kurian |
2005 | 32/F | PR | ITRT | NA | − | − | Alive | 15 mo |
| 37/F | Biopsy | ITRT | NA | Yes, 9 mo | PR | Died | 11 mo | |||
| 2 | Hanbali |
2001 | 57/M | PR | EBRT | 54 Gy | Yes, 7 mo | GTR | Died | 9 mo |
| 3 | Tonami |
2000 | 42/F | PR | EBRT; GKRS | EBRT 52 Gy; marginal 20 Gy | − | − | Alive | 9 mo |
| 4 | Reifenberger |
1999 | 56/M | PR | GKRS | NA | − | − | Alive | 42 mo |
| 5 | Kobayashi |
2013 | 55/F | Biopsy | GKRS | Marginal 11 Gy | − | − | − | − |
| 31/F | Biopsy | GKRS | Marginal 10.5 Gy | − | − | − | − | |||
| 61/F | PR | GKRS | Marginal 12 Gy | − | − | − | − | |||
| 6 | Nakajima |
2003 | 49/F | PR | GKRS | Marginal 12 Gy | − | − | Alive | 24 mo |
| 7 | Brat |
1998 | 59/M | PR | EBRT | NA | Yes, NA | − | − | 36 mo |
| 31/F | PR | EBRT | NA | − | − | Alive | 48 mo | |||
| 8 | Chung |
2007 | 48/M | Biopsy | GKRS | Marginal 18–20 Gy | Yes, 8 mo | GTR | Alive | 60 mo |
RT, radiotherapy; PR, partial resection; ITRT, intra-tumor radiotherapy; EBRT, external beam radiotherapy; GKRS, gamma-knife radiosurgery; NA, not available; GTR, gross total resection; mo, months; M, male; F, female.
In our case, only biopsy was performed before EBRT due to the location of the tumor and to the tight adhesion of the adjacent structure. SRS was not suitable for this case since the maximal diameter of the tumor prior to treatment was 53 mm. Therefore, EBRT was chosen as the treatment technique. The tumor was contoured based on 3 mm CT simulation images with contrast enhancement. A 3 mm margin was added to the GTV to create PTV. The dose to PTV was 62 Gy in 31 fractions. Here we chose a higher prescribed dose with a smaller PTV according to the literature reviewed. The earliest report from Brat et al. showed long term survival for two cases who received partial resection followed by EBRT, but the prescribed dose was not stated clearly (23). In GKRS series, a marginal dose of 10–12 Gy was often used after the biopsy to maintain a local control (20). Another case with a local control of 9 months received a boost of 54 Gy by EBRT after partial resection and GKRS (11 Gy marginally) (22). In another case, tumor recurrence was observed 7 months after partial resection and adjuvant EBRT of 54 Gy (24). It seemed reasonable for us that a higher dose was needed for this rare disease.
In general, a 1–2 cm margin is suggested outside of GTV to form a PTV for low-grade gliomas. Since our patient had only slurred speech before the radiotherapy, we wanted to maintain his neurological function as much as possible. To sum up, instead of treating the patient with 50–55 Gy with a 1–2 cm margin, we ended up treating him with 62 Gy with a 3 mm margin. The patient completed the treatment smoothly.
However, the regrowth of the tumor was observed 5 months after the completion of EBRT. In our case, the tumor regrows from the same location where it was treated heavily. The pattern of failure demonstrated in our case echoed to the cases reported in the literature. Yet again, emphasized the importance of possible resection before any type of radiotherapy started.
Symptoms of IICP including headache and vomiting have occurred after the tumor regrowth. Ten months after the radiotherapy, the patient died of urosepsis and multi-organ failure.
Conclusions
CG was a rare disease which arose from the thalamus or from the anterior wall of the third ventricle. GTR remained the mainstay of the treatment and should be considered in spite of all possibilities. The role of EBRT in this disease has not been fully known so far. For those cases who only received biopsy, the choice of definitive treatment is crucial yet limited. Our case report demonstrated that biopsy followed by EBRT may not be enough for long term control. To date, GKRS followed by biopsy with a marginal dose of 10–12 Gy may be a better choice than EBRT. If the tumor was not suitable for GKRS, EBRT with adequate dose and margins may be an option. Further investigations were needed to determine better treatment strategies for this disease.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2018.01.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kleihues P, Louis DN, Scheithauer BW, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol 2002;61:215-25; discussion 226-9. [Crossref] [PubMed]
- Morais BA, Menendez DF, Medeiros RS, et al. Chordoid glioma: Case report and review of the literature. Int J Surg Case Rep 2015;7C:168-71. [Crossref] [PubMed]
- Zarghouni M, Vandergriff C, Layton KF, et al. Chordoid glioma of the third ventricle. Proc (Bayl Univ Med Cent) 2012;25:285-6. [Crossref] [PubMed]
- Pasquier B, Péoc'h M, Morrison AL, et al. Chordoid glioma of the third ventricle: a report of two new cases, with further evidence supporting an ependymal differentiation, and review of the literature. Am J Surg Pathol 2002;26:1330-42. [Crossref] [PubMed]
- Pomper MG, Passe TJ, Burger PC, et al. Chordoid glioma: a neoplasm unique to the hypothalamus and anterior third ventricle. AJNR Am J Neuroradiol 2001;22:464-9. [PubMed]
- Liu WP, Cheng JX, Yi XC, et al. Chordoid glioma: a case report and literature review. Neurologist 2011;17:52-6. [Crossref] [PubMed]
- Ampie L, Choy W, Lamano JB, et al. Prognostic factors for recurrence and complications in the surgical management of primary chordoid gliomas: A systematic review of literature. Clin Neurol Neurosurg 2015;138:129-36. [Crossref] [PubMed]
- Johnson RR, Baehring J, Piepmeier J. Surgery for Third Ventricular Tumors. Neurosurg Quart 2003;13:207-25. [Crossref]
- Cenacchi G, Roncaroli F, Cerasoli S, et al. Chordoid glioma of the third ventricle: an ultrastructural study of three cases with a histogenetic hypothesis. Am J Surg Pathol 2001;25:401-5. [Crossref] [PubMed]
- Sanches P, Yamashita S, Freitas CC, et al. Chordoid glioma of the third ventricle: a new case report. Radiol Bras 2012;45:288-90. [Crossref]
- Kim JW, Kim JH, Choe G, et al. Chordoid glioma: a case report of unusual location and neuroradiological characteristics. J Korean Neurosurg Soc 2010;48:62-5. [Crossref] [PubMed]
- Tanboon J, Aurboonyawat T, Chawalparit O A. 29-year-old man with progressive short term memory loss. Brain Pathol 2014;24:103-6. [Crossref] [PubMed]
- Couce ME, Aker FV, Scheithauer BW. Chordoid meningioma: a clinicopathologic study of 42 cases. Am J Surg Pathol 2000;24:899-905. [Crossref] [PubMed]
- Buccoliero AM, Caldarella A, Gallina P, et al. Chordoid glioma: clinicopathologic profile and differential diagnosis of an uncommon tumor. Arch Pathol Lab Med 2004;128:e141-5. [PubMed]
- Reifenberger G, Weber T, Weber RG, et al. Chordoid glioma of the third ventricle: immunohistochemical and molecular genetic characterization of a novel tumor entity. Brain Pathol 1999;9:617-26. [Crossref] [PubMed]
- Vajtai I, Varga Z, Scheithauer BW, et al. Chordoid glioma of the third ventricle: confirmatory report of a new entity. Hum Pathol 1999;30:723-6. [Crossref] [PubMed]
- Desouza RM, Bodi I, Thomas N, et al. Chordoid glioma: ten years of a low-grade tumor with high morbidity. Skull Base 2010;20:125-38. [Crossref] [PubMed]
- Nakajima M, Nakasu S, Hatsuda N, et al. Third ventricular chordoid glioma: case report and review of the literature. Surg Neurol 2003;59:424-8. [Crossref] [PubMed]
- Chung SB, Park SH, Kim JE. Chordoid glioma of the third ventricle with unusual MRI features. J Korean Neurosurg Soc 2007;42:224-7.
- Kobayashi T, Tsugawa T, Hashizume C, et al. Therapeutic approach to chordoid glioma of the third ventricle. Neurol Med Chir (Tokyo) 2013;53:249-55. [Crossref] [PubMed]
- Kurian KM, Summers DM, Statham PF, et al. Third ventricular chordoid glioma: clinicopathological study of two cases with evidence for a poor clinical outcome despite low grade histological features. Neuropathol Appl Neurobiol 2005;31:354-61. [Crossref] [PubMed]
- Tonami H, Kamehiro M, Oguchi M, et al. Chordoid glioma of the third ventricle: CT and MR findings. J Comput Assist Tomogr 2000;24:336-8. [Crossref] [PubMed]
- Brat DJ, Scheithauer BW, Staugaitis SM, et al. Third ventricular chordoid glioma: a distinct clinicopathologic entity. J Neuropathol Exp Neurol 1998;57:283-90. [Crossref] [PubMed]
- Hanbali F, Fuller GN, Leeds NE, et al. Choroid plexus cyst and chordoid glioma. Report of two cases. Neurosurg Focus 2001;10:E5 [Crossref] [PubMed]
Cite this article as: Chou YH, Tseng HC, Lee YC. Chordoid glioma of the third ventricle—a case report and literature review. Ther Radiol Oncol 2018;2:8.


