Prognostic factors and treatment results of preoperative intra-arterial concurrent chemoradiation therapy in locally advanced head and neck cancer
Introduction
In early stage oral and oropharyngeal cancer without lymph node involvement, surgery or definitive radiotherapy is usually performed with curative intent. For advanced disease, surgery remains the standard treatment. Surgery for advanced disease often requires partial or complete resection of the larynx, pharynx, maxilla, tongue and buccal area which can have a detrimental impact on the patient’s speech and swallowing functions (1,2).
In 1965 in France, the first randomized controlled trial comparing chemo-radiotherapy with radiotherapy alone for oral cavity & oropharyngeal cancer was conducted. This was the first multi-modality treatment for advanced head and neck cancer and its results were encouraging. Tumor regression at the second month was 41% for combination therapy and 9% for radiotherapy alone. The survival benefit shown at the sixth and ninth months respectively disappeared after the 18th month. This called for a different combination of chemotherapeutic agents combined with radiotherapy (3).
Intra-arterial concurrent chemo-radiotherapy is an alternative treatment for patients with inoperable, locally advanced cancers, which allows organ preservation and maintains quality of life (2). The root of intra-arterial chemotherapy was an accidental injection of chlormethine (nitrogen mustard) into a brachial artery during the treatment of Hodgkin’s lymphoma in the 1950s. An initial local inflammation followed by vesiculation, and ulceration of the hand and forearm which subsided eventually without irreversible change (4). The result sparked interest in clinicians using this regional chemotherapy to treat cancers while circumventing the limitation of hematopoiesis suppression with intravenous chemotherapy.
With the advances in interventional radiology techniques and devices, superselective catheterization of the tumor feeding artery under the fluoroscopic guidance is feasible and a microcatheter can be left in place for chemotherapy. A phase II trial, conducted by Robbins et al., evaluated the response rate of weekly high dose cisplatin (150 mg/m2/week) for four weeks, via the transfemoral route, concurrent with conventional radiotherapy (66–74 Gy to gross nodal disease and 50 Gy to uninvolved lower neck, at 2.0 Gy per fraction) for patients with unresectable stage III–IV head and neck cancers. The weekly bolus injection of cisplatin is accompanied by a simultaneous intravenous sodium thiosulfate injection (9 g/m2) to neutralize the cisplatin in systemic circulation, thus minimizing the toxicity. The weekly supradose cisplatin with radiotherapy, RADPLAT regimen, attained a complete response rate of 75%, with acceptable toxicity and excellent local control (5). RTOG 9615 trial, conducted by Robbins et al. from 1997 to 1999, was employing a similar protocol of intra-arterial chemotherapy concurrent with radiotherapy (70 Gy, 2 Gy per fraction) for stage IV head and neck squamous cell carcinoma. In this trial, high-dose cisplatin was administered via external carotid route into tumor feeding arteries, rather than transfemoral route. This attained a similar overall complete response rate of 80%, with estimated 1-year and 2-year local control rates of 66% and 57%, respectively (6).
A different approach using the superficial temporal artery or occipital artery in a retrograde fashion was utilized by Mitsudo et al. to deliver concurrent chemotherapy of daily cisplatin (5 mg/m2/day) and weekly docetaxel (15 mg/m2/week). The complete response rate reached 100% after completion of chemo-radiotherapy and with estimated 1-year and 3-year control rates of 83.3%, 79.7%, respectively (7).
We retrospectively reviewed the results of 31 patients with advanced, inoperable oral cavity cancer, oropharyngeal cancer, and hypopharyngeal cancer treated with retrograde superselective intra-arterial chemo-radiotherapy via superficial temporal artery followed by surgical resection, if deemed operable by oral maxillofacial surgeon. This study was approved by institutional review board, document number 20161103B.
Methods
Patients
Patients with stage III and IV, inoperable, locally advanced oral cavity, oropharyngeal, and hypopharyngeal cancer treated with retrograde intra-arterial chemotherapy and daily concurrent radiotherapy were analyzed in this review. All patient has Eastern Cooperative Oncology Group (ECOG) score of less than or equal to 2. Patient’s age and co-morbidities were not considered in the inclusion criteria. Diagnoses were established with tissue biopsy and the patients were staged before the start of intra-arterial concurrent chemo-radiation therapy (IACCRT) with positron emission tomography computed tomography (PET-CT), or magnetic resonance imaging (MRI), or computed tomography (CT) and the size of the primary tumors were obtained. The stages of the diseases were determined according to American Joint Committee on Cancer (AJCC) TNM Staging Classification for the Lip and Oral Cavity, and Pharynx (7th ed., 2010). Toxicities were assessed with Common Terminology Criteria for Adverse Events, Version 4.0. The WHO criteria for treatment response were employed. PET-CT, MRI, or CT follow-up image study was arranged approximately 30 to 35 days after the end of chemoradiation therapy and compare with the reference image study prior to the treatment.
Treatment
Retrograde superselective superficial temporal artery catheterization
On the lesion side, the superficial temporal artery was catheterized with ultrasonographic guidance using the Seldinger technique with a 21G sheathed intravenous needle, and then a 4 or 5 Fr angiographic catheters was advanced over-the-wire into the external carotid artery. After that an arteriography was performed to determine the feeding artery and tumor vascularity. If the angiographic catheter could not be placed directly into the feeding artery, a 2.7 Fr microcatheter (Progreat, Terumo, Japan) would be placed coaxially through the angiographic catheter, into the feeding artery. If superselective catheterizations of the tumor feeding artery were not possible or there were multiple tumor feeding arteries, the catheter was positioned in the external carotid artery. After successful placement of the angiographic catheter or microcatheter, heparinized solution was maintained with a pump to avoid catheter occlusion.
Concurrent chemoradiotherapy via intra-arterial route
Thermoplastic masks were created to immobilize the patients. Radiotherapies were planned using conventional dose fraction of 54 Gy/27 fractions prior to 2013. After 2013, the protocol of simultaneous integrated boost (SIB) technique was adopted in which primary tumors received 37.5 Gy/15 fractions and neck lymph node levels I, II, III, IV, V received 30.0 Gy/15 fractions, then the angiographic catheter or microcatheter were removed. The primary tumor and regional neck area would receive radiotherapy alone with further boost of 16 Gy/8 fractions. The target volume for N0 to N2b disease encompassed the primary tumor and ipsilateral cervical lymph nodes, level I to level V. The target volume for N2c contained the primary tumor and bilateral cervical lymph nodes, level I to level V. The chemotherapy was administered via the superficial temporal artery route with cisplatin 10 mg/m2/day, 5 days a week, continuous infusion, therefore for protocol before 2013, a total of 270 mg/m2 was infused and total of 150 mg/m2 was infused for protocol after 2013. One month after the end of intra-arterial chemo-radiotherapy, the patient would be referred to an oral-facial surgeon for evaluation of surgery. Those who remained inoperable after evaluation by oral maxillofacial surgeon would receive adjuvant concurrent chemo-radiotherapy.
Statistical analysis
Locoregional-recurrence-free survival and overall survival was estimated with Kaplan-Meier methods. The differences between the treatment variables of the patients were assessed with log-rank trend tests for significance. Locoregional-recurrence-free survival was measured from beginning of radiotherapy until the day of locoregional recurrence, as noted on follow-up image studies. Overall survival was measured from the beginning of radiotherapy until the day of death. The Cox regression model was used for multivariate analysis. SPSS (SPSS Inc. Chicago, IL, USA) version 24.0 was used for data analysis.
Results
Treatment results
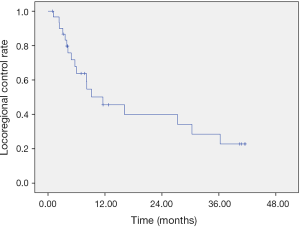
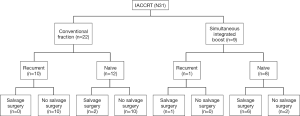
The primary tumor sites of the 31 patients were tongue (n=12), buccal (n=8), tonsil (n=4), hypopharynx (n=3), gingiva (n=2), and palate (n=2). Details of the patient characteristics are shown in Table 1 (TNM staging classifications in this table are a combination of newly diagnosed and recurrent disease). Twenty-eight patients had stage IV disease, while three patients were stage III. Among the 31 patients, 22 were treated with conventional doses and fractions, while 9 were treated with SIB technique. There were 10 (45%) recurrent cases (previously treated with radiotherapy) and 12 (55%) treatment-naïve cases within the conventional dose and fraction group. Among the 9 patients treated with SIB technique, only 1 (11%) patient was a recurrent case and 8 (89%) patients were treatment-naïve cases. After the intra-arterial concurrent chemo-radiotherapy, no salvage operations were feasible for the recurrent cases in the group of conventional radiotherapy dose and fraction. The salvage surgeries for the residual tumors were feasible only in 2 (17%) radiotherapy-naïve patients within the conventional radiotherapy group. The salvage operation of residual tumors was feasible for 7 out of 9 patients who underwent SIB technique. One patient had progressive disease despite treatment and the other patient refused surgical intervention even with partial response after the chemoradiation. The median follow-up time was 11.6 months. The details of the treatment results are on Figure 1.
Table 1
| Characteristics | No. of patients [%] |
|---|---|
| Gender | |
| Male | 27 [87] |
| Female | 4 [13] |
| Primary tumor site | |
| Tongue | 12 [39] |
| Buccal | 8 [26] |
| Tonsil | 4 [13] |
| Hypopharynx | 3 [10] |
| Gingiva | 2 [6] |
| Palate | 2 [6] |
| T classification | |
| T1 | 1 [3] |
| T2 | 5 [16] |
| T3 | 2 [6] |
| T4a | 20 [67] |
| T4b | 3 [10] |
| N classification | |
| N0 | 9 [29] |
| N1 | 4 [13] |
| N2a | 0 |
| N2b | 13 [42] |
| N2c | 1 [3] |
| N3 | 4 [13] |
| Stage classification | |
| III | 3 [10] |
| IVa | 25 [81] |
| IVb | 3 [10] |
| Total | 31 [100] |
All patients have ECOG score ≤2.
Tumor responses
All the patients were followed monthly in the first year and every 2 months in second year. Follow-up image studies were arranged approximately one month after the chemoradiation. Four patients did not return to out-patient clinic after the follow-up image study was taken. Complete responses were observed in 6 out of 31 (19%) cases, 4 from the conventional fraction group and 2 from the SIB group. Partial response was observed in 10 (32.0%) out of 31 patients, 7 from the conventional fraction group and 3 from the SIB group. The responders, consist of patients with complete response and partial response, from the conventional group were 11 (50%) out of 22 patients. The responders from the SIB group were 5 (56%) out of 9 patients. Almost all the cases with complete response and partial response are treatment-naïve cases, except one patient with complete response was a recurrent buccal caner. Majority of the non-responders, consist of stationary disease or progressive disease, were the recurrent cases.
Toxicities
Table 2 shows the toxicity reported in patients with conventional dose fraction and SIB techniques. The toxicity grading was assessed using Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Overall, there were no grade V toxicities in either group. There was one case of grade IV hematologic toxicity in each group. These two patients, who experienced anemia with neutropenic fever during the treatment were both transferred to intensive-care unit for monitoring, and recovered without further sequela. Grade III mucositis or dysphagia were found in around fifty percent of patients. One case of grade IV neurologic toxicity was found in the conventional dose fraction group. This patient experienced seizures as an IACCRT-related complication. There were no signs of infarction on the brain CT image and the follow-up brain MRI only noted slight brain atrophy. Although no permanent damage was observed in a brain image, the intra-arterial catheter was removed and the patient continued chemotherapy with left subclavian port-A implant. Systemic infections requiring intravenous antibiotics, CTCAE grade III, were observed in 7 (31.8%) and 5 (55.6%) patients in the conventional dose fraction group and the SIB group, respectively. Creatinine elevation over three times the baseline, CTCAE grade III, was not observed, but most patients had grade I acute kidney injuries.
Table 2
| Toxicity grade | No. of patients treated with conventional dose fraction | |||
|---|---|---|---|---|
| I | II | III | IV | |
| Hematologic (N=22) | 10 | 6 | 5 (22.7) | 1 (4.5) |
| Mucositis/dysphagia (N=22) | 1 | 10 | 11 (50.0) | 0 |
| Neurologic (N=22) | 18 | 2 | 1 (4.5) | 1 (4.5) |
| Infection (N=22) | 9 | 4 | 7 (31.8) | 2 (9.0) |
| Acute kidney injury (N=22) | 20 | 2 | 0 | 0 |
| Nausea/vomiting (N=22) | 6 | 13 | 3 (13.6) | 0 |
| Hematologic (N=9) | 2 | 5 | 1 (11.1) | 1 (11.1) |
| Mucositis/dysphagia (N=9) | 1 | 3 | 5 (55.6) | 0 |
| Neurologic (N=9) | 8 | 0 | 1 (1.1) | 0 |
| Infection (N=9) | 2 | 2 | 5 (55.6) | 0 |
| Acute kidney injury (N=9) | 8 | 1 | 0 | 0 |
| Nausea/vomiting (N=9) | 2 | 6 | 1 (11.1) | 0 |
Patient and treatment related variables
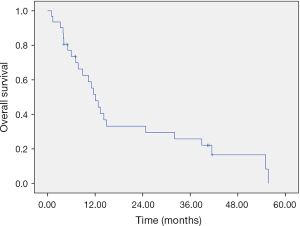
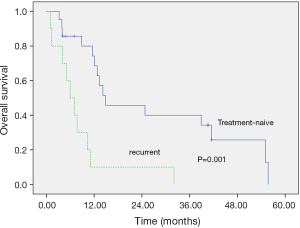
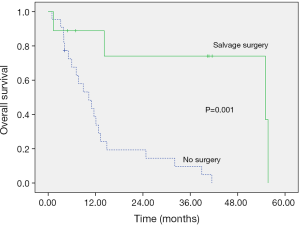
The locoregional-recurrence-free survival and overall survival rates were estimated using Kaplan-Meier methods, with median locoregional-recurrence-free survival of 11.57 months (Figure 2) and median overall survival of 12.10 months (Figure 3). The 2-year locoregional-recurrence-free survival is 37.1%, while 2-year overall survival rate is 31.3%. Differences in locoregional-recurrence-free survival and overall survival were tested for significance for variables in univariate analysis, such as radiotherapy technique (conventional fraction versus SIB), site of tumor (oral cavity vs. oropharynx vs. hypopharynx), status before treatment (recurrent vs. treatment-naive), salvage surgery (no surgery vs. surgery), and TNM staging (III vs. IVa vs. IVb) (Table 3). The result showed significance in locoregional-recurrence-free survival curve and overall survival curve for the status of the patient (recurrent vs. treatment-naïve), P=0.013 and P=0.001, respectively (Figures 4,5). Patient receiving salvage surgery or not is a significant variable for overall survival (Figure 6, P=0.038), but this is not the case in locoregional-recurrence-free survival. The significant variables in univariate analysis were then subject to Cox Regression for multivariate analysis (Table 4). The treatment-naïve patients had 0.38 times the risk of dying than patients with recurrent cancer (HR =0.382, P=0.038) and patients who did not receive salvage surgery had 6.61 times the risk of dying, then patients who received salvage surgery (HR =6.605, P=0.016).
Table 3
| Variables | LRC P value | OS P value |
|---|---|---|
| Tumor site (OC, OPX, HPX) | 0.673 | 0.992 |
| Status before treatment (treatment naïve |
0.013* | 0.001* |
| Radiotherapy techniques (SIB |
0.057 | 0.069 |
| Salvage surgery (no surgery |
0.118 | 0.001* |
| TNM stage (III |
0.597 | 0.282 |
*, statistically significant. LRC, locoregional control; OS, overall survival; OC, oral cavity; OPX, oropharynx; HPX, hypopharynx; SIB, simultaneous integrated boost.
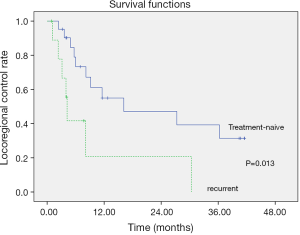
Table 4
| Variables | P value | HR | CI |
|---|---|---|---|
| Status before treatment (treatment-naïve |
0.038 | 0.382 | 0.153–0.950 |
| Salvage surgery (no surgery |
0.016 | 6.605 | 1.428–30.542 |
HR, hazard ratio; CI, confidence interval.
Discussion
Cisplatin, when administered systematically, is hampered by the toxic effect to vital organs such as the kidneys. Renal toxicity is cumulative but severity can be reduced by prehydration with intravenous fluid but could not be completely avoided (8). Nausea and vomiting are very common with systemic infusion. Ototoxicity is cumulative and irreversible. Neurotoxicity is irreversible in 30–50% of patients and with 300 mg/m2 cumulative doses; neurotoxicity may become noticeable and progress for months after stopping cisplatin (9). Even with adequate hydration, renal toxicity continues to be a limiting factor when considering infusing higher dose of cisplatin than 125 mg/m2 (10). Higher systemic doses may require subsequent infusion of sodium thiosulfate to decrease toxicity to the kidney. Another strategy to increase chemotherapeutic agent concentration within the tumor is via the intra-arterial route. Intra-arterial chemotherapy, when factored into the regional blood flow, drug delivery rate, and total body clearance may create a two to four-fold increase drug concentration within the tumor (11).
There are three methods of intra-arterial catheterization. Conventional intra-arterial infusion via superficial temporal artery, superselective intra-arterial infusion via femoral artery with Seldinger method, and the method we utilized, retrograde superselective intra-arterial infusion via superficial temporal artery. The conventional intra-arterial infusion via superficial temporal artery method, places the catheter within the external carotid artery. The procedure is easy to perform, however, chemotherapeutic agents infused may not reliably traverse the tumor, and the concentration of the chemotherapeutic agents may fluctuate on a daily basis. Unlike the superselective methods, which use a curved tip catheter, conventional method use a straight-tip catheter which may be displaced with neck flexion or extension (12). The superselective intra-arterial method via femoral artery or Seldinger method was used by Robbins et al. (13). This method allows infusion of the chemotherapeutic agent directly into the tumor-feeding artery without fluctuation of concentration of chemotherapeutic agent. However, this method is associated with cerebral infarction and sudden death as reported by Lee et al. (14). These severe complications are likely due to insertion of the catheter via the common carotid artery bifurcation each time a chemotherapeutic agent is injected. Clots at the common carotid artery bifurcation may be dislodged and travel into internal carotid artery and cause severe complications. Retrograde superselective intra-arterial method via superficial temporal artery overcomes the flaws mentioned above. A curved-tip catheter is used and inserted in retrograde direction, bypassing the common carotid artery bifurcation, and fixed in the tumor-feeding artery (15,16).
Prior to 2013, with the conventional dose fraction, there were few cases of catheter infection, displacement, and intra-catheter thrombus formation due to prolonged catheter placement. Therefore, after 2013, radiotherapy protocol was replaced with the SIB technique to decrease the dwelling time of the intra-arterial catheter from 5 to 3 weeks. A shortened dwelling time for the catheter is correlated with better patient compliance, and less chance of catheter infections and displacement.
In our search of literature, we found no description of combining neoadjuvant intra-arterial concurrent chemo-radiotherapy with salvage surgery. However, there are reports of neoadjuvant systemic chemotherapy combined with salvage surgery with very impressive overall and disease-free survival rates (17-19). Slotman et al. studied the effects of neoadjuvant chemoradiotherapy using cisplatin (20 mg/m2) on days 1 to 4 and 22 to 25 during the course of radiotherapy of 45 Gy in 25 fractions on operable stage III or IV squamous cell carcinoma of the head and neck patients. The study reported an overall response rate of 94% for primary tumors, 100% for cervical metastases, and a 2-year survival rate of about 65%, one of the most remarkable results among head and neck cancer treatment protocols (20-22). In our retrospective review, complete tumor response rate was 19%, partial tumor response rate was 32%, overall response rate, 51% and the 2-year survival rate was 31.3%. The vast differences between our results is likely due to the difference in severity of tumor involvement and the fact that 35.5% of patients in our study were recurrent cases and 91% of our patients were stage IV disease, while Slotman et al. reported 62% are stage III disease (19). Several recent studies reported even higher long-term survival of between 60% and 80%. However, these studies included stage II disease treated with multimodalities added into the analysis (23,24). A stage II oral squamous cell carcinoma treated with primary surgery or definitive radiotherapy may already have a 5-year overall survival rate of approximately 75% (25).
In this retrospective review, grade III mucositis experienced by patients undergoing conventional dose fractions or SIB were 50% and 55.6%, respectively. In a randomized phase 3 trial of 239 patients comparing conventional radiotherapy with either intra-arterial or intravenous cisplatin. There were 94% of the patients in the intra-arterial arm and 87% of patients in the intravenous arm who required tube feeding throughout the treatment. The number of patients requiring tube feeding was not significantly different between the intra-arterial and intravenous arm group. Nevertheless, in this phase 3 trial, intra-arterial chemotherapy was using the RADPLAT protocol, which requires administration of 150 mg/m2 on days 1, 8, 15 and 22, and the intravenous arm requires administration of 100 mg/m2 on Days 1, 22 and 43 (26). Both types in this phase 3 trial had significantly higher doses of cisplatin administered than in our protocol, consequently, more severe toxicity is to be expected. One should note that renal toxicity over grade II was more prominent in the intravenous arm than the intra-arterial arm (P<0.0001) in this phase 3 study, thus providing evidence that intra-arterial chemotherapy could dramatically reduce renal toxicity. Furthermore, another study, a phase 2 trial, which experimented using low dose, intravenous, 6 mg/m2 for 20 cycles of cisplatin, resulted in a higher rate of grade 3 mucositis of 65% and grade II renal toxicity of 22% compared to our protocol of intra-arterial chemotherapy (8). In our patients, 90% of the patients had only grade 1 renal toxicity. Hematological toxicity was also more prominent in this phase II trial compared to ours, with mainly leucopenia (23% grade 3 and 9% grade 4); while we had 2 patients with grade 4 and 6 patients with grade 3 hematological toxicity.
The status of the disease before treatment (recurrent vs. treatment-naïve) and whether or not the patient underwent salvage surgery were independent prognostic factors. However, there was no significant difference between locoregional-recurrence-free survival and overall survival between the two different radiotherapy techniques, P=0.057 and P=0.069, respectively. We did, however, observe a trend of improved locoregional-recurrence-free survival and overall survival with the SIB technique.
The limitation of this study is that this is a retrospective review of a single institution; therefore, bias and overlap are implicit. Heterogeneity of the patient treatment status (recurrent vs. treatment naïve) before IACCRT is another limitation since re-irradiation of the second primary or recurrent head and neck cancer could be limited by the previous radiation dose, if unresectable. This gives a relatively low response rate (20–35%) to chemotherapy (27).
In conclusion, preoperative intra-arterial concurrent chemo-radiotherapy with salvage surgery offers a feasible, low toxicity treatment alternative since the mean radiation dose administered is lower than most postoperative protocols (28,29). In this study, SIB technique converted 75% of the inoperable tumor into operable cases, while conventional dose fraction was only capable of 16% conversion to operable tumor if we only analyze the result from treatment-naïve patients. Our approach may potentially offer a chance to convert inoperable locally advanced head and neck cancer into operable cases, and therefore confers longer overall survival, mainly through surgical resection (30). Although SIB technique did not significantly improve the locoregional-recurrence-free survival and overall survival rate compared to conventional dose fraction, such trends do exist. Further prospective randomized studies with more patient accrual are needed to assess the effectiveness of SIB technique in reducing the tumor volume and extension to a degree suitable for surgery and whether or not intra-arterial chemotherapy is as effective as the intravenous route and with minimal side effects.
Acknowledgments
Funding: This investigation was supported by grants from the Yuan’s General Hospital (YGH-17-001).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by institutional review board, document number 20161103B. Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Deleyiannis FW, Weymuller EA Jr, Coltrera MD. Quality of life of disease-free survivors of advanced (stage III or IV) oropharyngeal cancer. Head Neck 1997;19:466-73. [Crossref] [PubMed]
- Mitsudo K, Koizumi T, Iida M, et al. Retrograde superselective intra-arterial chemotherapy and daily concurrent radiotherapy for stage III and IV oral cancer: analysis of therapeutic results in 112 cases. Radiother Oncol 2014;111:306-10. [Crossref] [PubMed]
- Richard JM, Sancho H, Lepintre Y, et al. Intra-arterial methotrexate chemotherapy and telecobalt therapy in cancer of the oral cavity and oropharynx. Cancer 1974;34:491-6. [Crossref] [PubMed]
- Klopp CT, Alford TC, Bateman J, et al. Fractionated intra-arterial cancer; chemotherapy with methyl bis amine hydrochloride; a preliminary report. Ann Surg 1950;132:811-32. [Crossref] [PubMed]
- Robbins KT, Kumar P, Regine WF, et al. Efficacy of targeted supradose cisplatin and concomitant radiation therapy for advanced head and neck cancer: the Memphis experience. Int J Radiat Oncol Biol Phys 1997;38:263-71. [Crossref] [PubMed]
- Robbins KT, Kumar P, Harris J, et al. Supradose intra-arterial cisplatin and concurrent radiation therapy for the treatment of stage IV head and neck squamous cell carcinoma is feasible and efficacious in a multi-institutional setting: results of Radiation Therapy Oncology Group Trial 9615. J Clin Oncol 2005;23:1447-54. [Crossref] [PubMed]
- Mitsudo K, Shigetomi T, Fujimoto Y, et al. Organ preservation with daily concurrent chemoradiotherapy using superselective intra-arterial infusion via a superficial temporal artery for T3 and T4 head and neck cancer. Int J Radiat Oncol Biol Phys 2011;79:1428-35. [Crossref] [PubMed]
- Hoebers FJ, Heemsbergen W, Balm AJ, et al. Concurrent chemoradiation with daily low dose cisplatin for advanced stage head and neck carcinoma. Radiother Oncol 2007;85:42-7. [Crossref] [PubMed]
- Ahn MJ, D’Cruz A, Vermorken JB, et al. Clinical recommendations for defining platinum unsuitable head and neck cancer patient populations on chemoradiotherapy: A literature review. Oral Oncol 2016;53:10-6. [Crossref] [PubMed]
- Robbins KT, Homma A. Intra-arterial chemotherapy for head and neck cancer: experiences from three continents. Surg Oncol Clin N Am 2008;17:919-33. [Crossref] [PubMed]
- Muchmore JH, Wanebo HJ. Regional chemotherapy: overview. Surg Oncol Clin N Am 2008;17:709-30. vii. [Crossref] [PubMed]
- Duff JK, Sullivan RD, Miller E, et al. Antimetabolite-metabolite cancer chemotherapy using continuous intra-arterial methotrexate with intermittent intramuscular citrovorum factor. Method of therapy. Cancer 1961;14:744-52. [Crossref] [PubMed]
- Robbins KT, Storniolo AM, Kerber C, et al. Phase I study of highly selective supradose cisplatin infusions for advanced head and neck cancer. J Clin Oncol 1994;12:2113-20. [Crossref] [PubMed]
- Lee YY, Dimery IW, Van Tassel P, et al. Superselective intra-arterial chemotherapy of advanced paranasal sinus tumors. Arch Otolaryngol Head Neck Surg 1989;115:503-11. [Crossref] [PubMed]
- Iwai T, Fuwa N, Hirota M, et al. Secure surgical method for catheter placement via the occipital artery to achieve retrograde superselective intra-arterial chemotherapy for advanced oral cancer: alternative to approach via the superficial temporal artery. Indian J Otolaryngol Head Neck Surg 2014;66:205-7. [Crossref] [PubMed]
- Tohnai I, Fuwa N, Hayashi Y, et al. New superselective intra-arterial infusion via superficial temporal artery for cancer of the tongue and tumour tissue platinum concentration after carboplatin (CBDCA) infusion. Oral Oncol 1998;34:387-90. [Crossref] [PubMed]
- Eckardt A, Sinikovic B, Hofele C, et al. Preoperative paclitaxel/carboplatin radiochemotherapy for stage III/IV resectable oral and oropharyngeal cancer: seven-year follow-up of a phase II trial. Oncology 2007;73:198-203. [Crossref] [PubMed]
- Kirita T, Ohgi K, Shimooka H, et al. Preoperative concurrent chemoradiotherapy plus radical surgery for advanced squamous cell carcinoma of the oral cavity: an analysis of long-term results. Oral Oncol 1999;35:597-606. [Crossref] [PubMed]
- Slotman GJ, Doolittle CH, Glicksman AS. Preoperative combined chemotherapy and radiation therapy plus radical surgery in advanced head and neck cancer. Five-year results with impressive complete response rates and high survival. Cancer 1992;69:2736-43. [Crossref] [PubMed]
- Jacobs JR, Fu KK, Lowry LD, et al. 5-year results of cisplatin and fluorouracil infusion in head and neck cancer. Arch Otolaryngol Head Neck Surg 1991;117:288-91. [Crossref] [PubMed]
- Rentschler RE, Wilbur DW, Petti GH, et al. Adjuvant methotrexate escalated to toxicity for resectable stage III and IV squamous head and neck carcinomas--a prospective, randomized study. J Clin Oncol 1987;5:278-85. [Crossref] [PubMed]
- Taylor SG 4th, Murthy AK, Caldarelli DD, et al. Combined simultaneous cisplatin/fluorouracil chemotherapy and split course radiation in head and neck cancer. J Clin Oncol 1989;7:846-56. [Crossref] [PubMed]
- Klug C, Wutzl A, Kermer C, et al. Preoperative radiochemotherapy and radical resection for stages II-IV oral and oropharyngeal cancer: outcome of 222 patients. Int J Oral Maxillofac Surg 2005;34:143-8. [Crossref] [PubMed]
- Wanebo HJ, Chougule P, Akerley WL, et al. Preoperative chemoradiation coupled with aggressive resection as needed ensures near total control in advanced head and neck cancer. Am J Surg 1997;174:518-22. [Crossref] [PubMed]
- Pfister DG, Ang K, Brockstein B, et al. NCCN practice guidelines for head and neck cancers. Oncology (Williston Park) 2000;14:163-94. [PubMed]
- Rasch CR, Hauptmann M, Schornagel J, et al. Intra-arterial versus intravenous chemoradiation for advanced head and neck cancer: Results of a randomized phase 3 trial. Cancer 2010;116:2159-65. [Crossref] [PubMed]
- Wong SJ, Machtay M, Li Y. Locally recurrent, previously irradiated head and neck cancer: concurrent re-irradiation and chemotherapy, or chemotherapy alone? J Clin Oncol 2006;24:2653-8. [Crossref] [PubMed]
- Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004;350:1945-52. [Crossref] [PubMed]
- Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004;350:1937-44. [Crossref] [PubMed]
- Lung T, Tăşcău OC, Almăşan HA, et al. Head and neck cancer, treatment, evolution and post therapeutic survival - Part 2: a decade's results 1993-2002. J Craniomaxillofac Surg 2007;35:126-31. [Crossref] [PubMed]
Cite this article as: Yuan TZ, Wang CY, Leung SW, Pai LC, Chen MCY, Leung KW, Huang GC. Prognostic factors and treatment results of preoperative intra-arterial concurrent chemoradiation therapy in locally advanced head and neck cancer. Ther Radiol Oncol 2018;2:6.