
Prognostic effects of the metabolic tumor volume and total lesion glycolysis in patients with advanced squamous cell carcinoma of head and neck
Introduction
Head and neck cancer is the sixth most common malignancy worldwide and accounts for the fourth in man in Taiwan (1). These tumors are almost always histopathologically identical (>90% are squamous cell carcinoma) but their clinical behaviors and prognosis are quite heterogenous. Many studies tried to identify the high-risk clinicopathological features to predict the prognostic outcome, such as anatomic site, tumor size and extent of invasion, neck lymph node stage, poor pathologic characteristics (margin status, angiolymphatic invasion, perineural invasion and extracapsular extension, etc.), but these factors could not fully predict the patients’ prognosis (2,3).
18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) scan is a functional image reflecting the tumor activity based on glucose metabolism. The FDG-PET has become the essential initial staging tool for head and neck cancers. Allal et al. used cut-off value as 5.5 for the SUVmax of the primary tumor (> and ≤5.5) and reported that the high pre-treatment standardized uptake value was associated with worse disease-free survival (DFS) (3-year DFS was 42% vs. 79%, P=0.005) and worse local control (3-year local control was 55% vs. 86%, P=0.01) for head and neck cancer treated by radiotherapy with or without chemotherapy (4). As the evolution of clinical investigations for FDG-PET quantitative parameters, the most widely used parameter is the maximum standardized uptake value (SUVmax) of the tumor, which has been demonstrated to predict survival in head and neck cancers (4-7). Liao et al. reported that the pre-treatment SUVmax of the primary tumor was significantly associated with local control (5-year local control rate for cutoff value of 19.3 was 55% vs. 88%, P=0.0135) and could be one component in predicting overall survival (OS) when combination with other prognosticators (such as tumor invasion depth, nodal SUVmax and presence of pathologic lymph node metastases) in oral cavity squamous cell carcinoma treated with surgery and neck dissection (5,6). Lin et al. also demonstrated that the pre-treatment SUVmax of the primary tumor was significantly associated with the primary tumor relapse-free survival in oropharyngeal and hypopharyngeal cancer treated with radiotherapy (SUVmax of the primary tumor >11 had inferior 2-year primary tumor relapse-free survival, 41% vs. 75%, P=0.003) (7). However, no definite threshold of the SUVmax of the primary tumor has been validated and it begs a question that whether a single pixel value within tumor mass, like SUVmax, is a good prognostic factor representing the whole lesion.
Recently, the volume-based parameters, such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG) are theoretically better parameters estimating the metabolic tumor burden and have been demonstrated as better prognostic predictors than the SUVmax (8-12). Systemic review by Castelli (a total of 2,928 patients in 45 studies) reported that the MTV was well correlated with OS and DFS, with a higher predictive value than the SUVmax in head and neck cancer (13). Another systemic review by Pak (13 studies with a total of 1,180 patients) showed both the high volumetric parameters of the MTV and the TLG were associated with increased adverse events (progression or recurrence) and decreased OS [combined HR for adverse events was 3.06 (2.33–4.01, P<0.00001) with MTV and 3.10 (2.27–4.24, P<0.00001)] with TLG; the pooled HR for OS was 3.51 (2.62–4.72, P<0.00001) with MTV and 3.14 (2.24–4.40, P<0.00001) with TLG) (14). However, the enrolled patients in these studies had various kinds of primary tumor sites, all stage diseases with distinct prognosis, and treated with inconsistent treatment modalities.
Recent study published by Yu showed that the reduction percentage between the pre- and post-treatment MTV and TLG (42% reduction in MTV and 55% reduction in TLG) were correlated with event-free survival for head and neck cancer patients treated with induction chemotherapy (IndCT) with TPF regimen (Taxotere, Cisplatin and Fluorouracil) (15). The aim of this study is to explore the prognostic significance of the pre-treatment FDG-PET parameters on patients with locally advanced squamous cell carcinoma of the head and neck (SCCHN) who received IndCT followed by local therapy (surgery, radiotherapy or both).
Methods
Patients
From September 2010 to July 2013, a total of 50 patients with previously untreated, biopsy-proven SCCHN and stage III/IV diseases were enrolled to this retrospective study. The patient characteristics were described as Table 1. The median age at diagnosis was 52 years old (range, 35 to 77 years) and 92% of patients were male. Most patients (98%) belong to stage IV diseases. The numbers of the primary tumor origin were 13 in the oral cavity, 19 in the oropharynx and 18 in the hypopharynx, respectively.
Table 1
| Variable | N | % | SUVmax of the primary tumor | P | MTV | P | TLG | P | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (<22) | High (≥22) | Low (<85) | High (≥85) | Low (<620) | High (≥620) | |||||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |||||||||
| Sex | 0.9999 | 0.5843 | 0.9999 | |||||||||||||||||
| Women | 4 | 8.0 | 4 | 9.3 | 0 | 0.0 | 2 | 5.9 | 2 | 12.5 | 3 | 8.6 | 1 | 6.7 | ||||||
| Men | 46 | 92.0 | 39 | 90.7 | 7 | 100.0 | 32 | 94.1 | 14 | 87.5 | 32 | 91.4 | 14 | 93.3 | ||||||
| Age | 0.9999 | 0.7635 | 0.9999 | |||||||||||||||||
| <50 | 21 | 42.0 | 18 | 41.9 | 3 | 42.9 | 15 | 44.1 | 6 | 37.5 | 15 | 42.9 | 6 | 40.0 | ||||||
| ≥50 | 29 | 58.0 | 25 | 58.1 | 4 | 57.1 | 19 | 55.9 | 10 | 62.5 | 20 | 57.1 | 9 | 60.0 | ||||||
| Performance status | 0.6181 | 0.0017 | 0.0180 | |||||||||||||||||
| 0 | 4 | 8.0 | 4 | 9.3 | 0 | 0.0 | 0 | 0.0 | 4 | 25.0 | 1 | 2.9 | 3 | 20.0 | ||||||
| 1 | 38 | 76.0 | 33 | 76.7 | 5 | 71.4 | 26 | 76.5 | 12 | 75.0 | 26 | 74.3 | 12 | 80.0 | ||||||
| 2 | 8 | 16.0 | 6 | 14.0 | 2 | 28.6 | 8 | 23.5 | 0 | 0.0 | 8 | 22.9 | 0 | 0.0 | ||||||
| Differentiation | 0.9999 | 0.7872 | 0.9493 | |||||||||||||||||
| Well differentiation | 2 | 4.0 | 2 | 4.7 | 0 | 0.0 | 2 | 5.9 | 0 | 0.0 | 2 | 5.7 | 0 | 0.0 | ||||||
| Moderate differentiation | 24 | 48.0 | 20 | 46.5 | 4 | 57.1 | 16 | 47.1 | 8 | 50.0 | 17 | 48.6 | 7 | 46.7 | ||||||
| Poorly differentiation | 20 | 40.0 | 17 | 39.5 | 3 | 42.9 | 14 | 41.2 | 6 | 37.5 | 13 | 37.1 | 7 | 46.7 | ||||||
| Unknown | 4 | 8.0 | 4 | 9.3 | 0 | 0.0 | 2 | 5.9 | 2 | 12.5 | 3 | 8.6 | 1 | 6.7 | ||||||
| Primary site | 0.3977 | 0.2031 | 0.0875 | |||||||||||||||||
| Oral cavity | 13 | 26.0 | 10 | 23.3 | 3 | 42.9 | 7 | 20.6 | 6 | 37.5 | 8 | 22.9 | 5 | 33.3 | ||||||
| Oropharynx | 19 | 38.0 | 16 | 37.2 | 3 | 42.9 | 12 | 35.3 | 7 | 43.8 | 11 | 31.4 | 8 | 53.3 | ||||||
| Hypopharynx | 18 | 36.0 | 17 | 39.5 | 1 | 14.3 | 15 | 44.1 | 3 | 18.8 | 16 | 45.7 | 2 | 13.3 | ||||||
| Clinical T stage | 0.4717 | 0.2127 | 0.2610 | |||||||||||||||||
| T1 | 1 | 2.0 | 1 | 2.3 | 0 | 0.0 | 0 | 0.0 | 1 | 6.3 | 0 | 0.0 | 1 | 6.7 | ||||||
| T2 | 8 | 16.0 | 8 | 18.6 | 0 | 0.0 | 7 | 20.6 | 1 | 6.3 | 7 | 20.0 | 1 | 6.7 | ||||||
| T3 | 13 | 26.0 | 10 | 23.3 | 3 | 42.9 | 10 | 29.4 | 3 | 18.8 | 10 | 28.6 | 3 | 20.0 | ||||||
| T4 | 28 | 56.0 | 24 | 55.8 | 4 | 57.1 | 17 | 50.0 | 11 | 68.8 | 18 | 51.4 | 10 | 66.7 | ||||||
| Clinical N stage | 0.9999 | 0.0728 | 0.9999 | |||||||||||||||||
| N0 | 2 | 4.0 | 2 | 4.7 | 0 | 0.0 | 2 | 5.9 | 0 | 0.0 | 2 | 5.7 | 0 | 0.0 | ||||||
| N1 | 3 | 6.0 | 3 | 7.0 | 0 | 0.0 | 2 | 5.9 | 1 | 6.3 | 2 | 5.7 | 1 | 6.7 | ||||||
| N2 | 42 | 84.0 | 35 | 81.4 | 7 | 100.0 | 30 | 88.2 | 12 | 75.0 | 29 | 82.9 | 13 | 86.7 | ||||||
| N3 | 3 | 6.0 | 3 | 7.0 | 0 | 0.0 | 0 | 0.0 | 3 | 18.8 | 2 | 5.7 | 1 | 6.7 | ||||||
| Clinical stage | 0.9999 | 0.9999 | 0.9999 | |||||||||||||||||
| Stage III | 1 | 2.0 | 1 | 2.3 | 0 | 0.0 | 1 | 2.9 | 0 | 0.0 | 1 | 2.9 | 0 | 0.0 | ||||||
| Stage IV | 49 | 98.0 | 42 | 97.7 | 7 | 100.0 | 33 | 97.1 | 16 | 100.0 | 34 | 97.1 | 15 | 100.0 | ||||||
SUVmax, maximum standardized uptake value; MTV, metabolic tumor volume; TLG, total lesion glycolysis; ECOG, Eastern Cooperative Oncology Group.
Treatments
All patients received IndCT first with weekly CDFLEM regimen, consisting of cisplatin 60 mg/m2, day 1, docetaxel 50 mg/m2, day 8, 5-fluorouracil 2,500 mg/m2 plus leucovorin 250 mg/m2, day 15, and epirubicin 30 mg/m2 plus methotrexate 30 mg/m2, day 22, repeated every 4 weeks for 3–4 cycles. Local therapy after IndCT included surgery, radiotherapy (RT), concurrent chemoradiotherapy (CCRT), or cetuximab-radiotherapy (Bio-RT). The surgery is strongly recommended for oral cavity cancer patients, but alternative local therapy would be selected if they refused surgery. CCRT is indicated for patients who had no response to IndCT and RT alone for those who responsed to IndCT. Bio-RT is indicated for non-oral cavity cancer patients who had age ≥70 years, poor renal function and hearing impairment.
Seven of 13 patients with oral cavity cancer received surgery after IndCT. Two of them also received postoperative adjuvant RT due to pathologic risk features (one ypT4aN0, margin <1 mm; and one ypT1N2b, margin involved) and the remaining 5 patients did not receive postoperative adjuvant therapy [3 pathological complete response, 1 pathological stage T2N0, 1 refusal (ypT4aN0, margin 2 mm)].
Other patients with oropharynx (n=19) and hypopharynx (n=18) cancer and 6 oral cavity cancer patients received non-surgical local therapy modality, including intensity-modulated radiotherapy (IMRT) alone (n=24), Bio-RT (n=17), and CCRT (n=2), respectively. We used IMRT technique with conventional fractionation of a total 70 Gy (2.0 Gy daily fraction, five days per week) to the gross tumor volume region shown in pre-treatment CT scan or MRI (before IndCT). The drug of Bio-RT consisted of cetuximab, loading dose 400 mg/m2 day 1 and then 250 mg/m2 once per week. The concurrent chemotherapy during IMRT was weekly cisplatin 30 mg/m2.
18F-FDG-PET scan protocol
The pre-treatment FDG-PET scan was usually performed within 1 week prior to the start of the IndCT (median 5 days). All patients were asked to fast for a minimum of six hours (except for water) before the FDG-PET study. Their serum glucose levels were checked to ensure the readings were lower than 200 mg/dL before administration of the radiotracer. PET/CT images were acquired about 1 hour after intravenous injection of 3.7 MBq/kg body weight of F-18FDG.
The imaging was performed using a whole body GEMINI TF 16-slice PET/CT scanner (Philips Healthcare, Cleveland, OH, USA). CT images were obtained without injection of contrast medium using the following settings: 120 kVp, 50 mA, 5 mm slice thickness, and 5 mm intervals. PET images were performed from the head to the upper thigh, and then were reconstructed using vendor supplied ASTONISH time-of-flight iterative reconstruction (Philips Healthcare, Andover, MA). Attenuation correction was performed based on the CT data. The reconstructed spatial resolution was 5 mm in the centre of the field of view. The image voxel size was 4 mm × 4 mm × 4 mm for the PET images and 1.17 mm × 1.17 mm × 1.5 mm for the CT images.
Images analysis
PET images were retrospectively interpreted by two experienced nuclear medicine physicians by Philips Extended Brilliance Workspace Nuclear Medicine version 2.0. Tumor tracking was used to draw regions of interest. For semiquantitative analysis of FDG uptake, regions of interest were defined on the target lesions (primary tumor and metastatic neck lymph nodes) on the trans-axial PET images. The SUV was calculated for quantitative analysis of tumor FDG uptake, as follows:
SUV = tissue activity concentration (kBq/mL)/injected activity (kBq)/body weight (kg) with injected activity decay-corrected from the delay between injection and image acquisition.
Measurement of tumor volume
All tumor volume design was performed in the workstation SYNTEGRA (Phillips Medical Systems). The MTV was measured from attenuation-corrected FDG-PET images. The boundaries of the primary tumor and metastatic regional lymph nodes were drawn in the axial, coronal, and sagittal FDG-PET/CT images. To define the volume boundaries of the interest target, an SUV of 2.5 was assigned and the voxels with SUV intensity ≥2.5 were incorporated into the MTV (10,16). This volume was automatically determined within the region of interests by the review software. The average SUV (SUVmean) and maximum SUV (SUVmax) for the region with SUV ≥2.5 was also determined.
The TLG was calculated as SUVmean × MTV (10). The TLG values were divided into the TLG of primary tumor (primary TLG), TLG of the metastatic neck nodes (nodal TLG), and total tumor TLG (sum of the primary TLG plus nodal TLG), respectively.
Determination of the cut-off values for PET parameters
The receiver operating characteristic (ROC) curve and area under curve (AUC) were used to find the best cut-off values of the SUVmax of the primary tumor, the MTV and the TLG. The total tumor TLG was used as one of the quantitative FDG-PET parameters in our study. We obtained cut-off values as 22 for the SUVmax of the primary tumor (≥ and <22), 85 for the MTV (≥ and <85) and 620 for the TLG (≥ and <620), respectively for further survival analysis.
Follow-up and statistical analysis
Patients received post-treatment regular follow-up (physical examinations, routine complete blood cell count, serologic biochemistry data of liver and kidney, and fiberscope) monthly for 3–6 months, at 3-month intervals for 3 years, and every 4–6 months thereafter. The computed tomography of head and neck was performed every 3–6 months for 3 years and annually thereafter. Locoregional failure was defined as biopsy-proven recurrence or progression of disease on serial image studies after completion of radiotherapy.
OS was defined as the time between the start day of IndCT and death or last follow-up visit. Locoregional progression-free survival (LRPFS) and distant metastasis failure-free survival (DMFFS) were calculated from the start day of IndCT to the date of locoregional recurrence and distant metastasis, respectively. Survival curves were analyzed by Kaplan-Meier method and a log-rank test was used to compare the difference between the subgroups.
The univariate Cox proportional hazards regression model was adjusted by age (< or ≥50 years old), sex, Eastern Cooperative Oncology Group performance status (ECOG PS 0 vs. 1–2), primary tumor site (oral cavity vs. oropharynx vs. hypopharynx), T-stage (T1–3 vs. T4), N-stage (N0–1 vs. N2–3) and different quantitative FDG-PET parameters (SUVmax of the primary tumor, MTV and TLG). Cox proportional hazard model was used in multivariate analysis to identify independent prognostic factors. All statistical analyses were performed in SAS version 9.3 and a 2-sided P<0.05 was considered statistically significant.
Results
Treatment outcomes
After a median follow-up of 44 months (range, 7 to 69 months) for living patients, 11 patients experienced locoregional recurrence alone, 1 distant metastasis alone, and 2 failures in both sites. The median duration was 9 months (range, 7 to 25 months) from treatment to locoregional failure and 7 months (range, 7 to 40 months) from treatment to distant metastasis, respectively. So far, there were 16 deaths. The causes of death were 11 uncontrolled tumors, 3 non-cancer related, 1 complication-related and 1 unknown, respectively. The 3-year and 5-year OS rates were 66.7% and 63.8%, respectively. The 3-year and 5-year LRPFS rates were both 70.4%. The 3-year and 5-year DMFFS rates were 95.8% and 92.3%, respectively.
18F-FDG-PET parameters
The patient characteristics and their relationship with the 18F-FDG-PET parameters (SUVmax of primary tumor, MTV and TLG) were also described on Table 1. The median SUVmax of primary tumor for entire cohort was 15.5 (range, 4.8–55.7). Seven patients (14%) had primary tumor SUVmax ≥22, and all of them were clinical T3–4 tumor. The median MTV was 55.2 (range, 6.3–324.9). Sixteen patients (32%) had MTV ≥85, and most of them had clinical T4 (11/16, 68.8%) and clinical N2 (12/16, 75.0%) diseases. Fifteen patients (30%) had TLG ≥620, and the majority was clinical T4 (10/15, 66.7%) and clinical N2 (13/15, 86.7%) diseases. All of these patients who had higher value of PET-parameters had clinical stage IV disease.
Univariate analysis
The MTV and the TLG were the significant predictors for OS (P=0.0272 and 0.0185, respectively) and LRPFS (P=0.0346 and 0.0185, respectively) by univariate analysis (Tables 2,3). The other clinicopathological factors, such as sex, age, PS, primary site, T-stage, N-stage or the SUVmax of the primary tumor revealed no significant effects on both OS and LRPFS. No significant predictors were found in DMFFS.
Table 2
| Variable | Interpretation | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| SUVmax of primary tumor | High (≥22) vs. low (<22) | 1.494 | (0.426–5.245) | 0.5310 | – | – | – | |
| Metabolic tumor volume | High (≥85) vs. low (<85) | 3.060 | (1.134–8.255) | 0.0272 | 3.060 | (1.134–8.255) | 0.0272 | |
| Total lesion glycolysis | High (≥620) vs. low (<620) | 3.286 | (1.221–8.846) | 0.0185 | 3.286 | (1.221–8.846) | 0.0185 | |
| Age | ≥50 vs. <50 | 1.133 | (0.421–3.048) | 0.8041 | – | – | – | |
| Sex | Men vs. women | 0.717 | (0.163–3.161) | 0.6605 | – | – | – | |
| Performance status (ECOG) | 1–2 vs. 0 | 1.429 | (0.324–6.294) | 0.6370 | – | – | – | |
| Primary site | Oropharynx vs. oral cavity | 1.495 | (0.488–4.584) | 0.4815 | – | – | – | |
| Hypopharynx vs. oral cavity | 0.504 | (0.120–2.110) | 0.3482 | – | – | – | ||
| T-stage | T4 vs. T1–3 | 0.969 | (0.360–2.605) | 0.9501 | – | – | – | |
| N-stage | N2–3 vs. N0–1 | 1.906 | (0.252–14.437) | 0.5325 | – | – | – | |
ECOG, Eastern Cooperative Oncology Group.
Table 3
| Variable | Interpretation | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| SUVmax of primary tumor | High (≥22) vs. low (<22) | 1.241 | (0.275–5.599) | 0.7791 | – | – | – | |
| Metabolic tumor volume | High (≥85) vs. low (<85) | 3.284 | (1.090–9.896) | 0.0346 | 3.284 | (1.090–9.896) | 0.0346 | |
| Total lesion glycolysis | High (≥620) vs. low (<620) | 3.790 | (1.250–11.488) | 0.0185 | 3.790 | (1.250–11.488) | 0.0185 | |
| Age | ≥50 vs. <50 | 1.384 | (0.452–4.240) | 0.5694 | – | – | – | |
| Sex | Men vs. women | 1.096 | (0.142–8.445) | 0.9297 | – | – | – | |
| Performance status (ECOG) | 1–2 vs. 0 | – | – | – | – | – | – | |
| Primary site | Oropharynx vs. oral cavity | 0.582 | (0.130–2.604) | 0.4793 | – | – | – | |
| Hypopharynx vs. oral cavity | 1.166 | (0.329–4.136) | 0.8117 | – | – | – | ||
| T Stage | T4 vs. T1–3 | 2.528 | (0.695–9.201) | 0.1593 | – | – | – | |
| N stage | N2–3 vs. N0–1 | 1.657 | (0.215–12.756) | 0.6276 | – | – | – | |
ECOG, Eastern Cooperative Oncology Group.
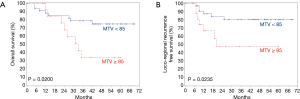
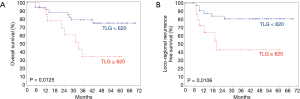
The 3-year rates of OS and LRPFS for patients with pre-treatment MTV < vs. ≥85 were 78.8% vs. 33.9% (P=0.0200, Figure 1A) and 80.2% vs. 47.6% (P=0.0235, Figure 1B). The corresponding rates for patients with pre-treatment TLG < vs. ≥620 were 78.8% vs. 34.6% (P=0.0125, Figure 2A) and 80.9% vs. 43.1% (P=0.0106, Figure 2B).
Multivariate analysis
Multivariate analysis showed that the MTV and TLG were independent factors in predicting OS (P=0.0272 and 0.0185, respectively) and LRPFS (P=0.0346 and 0.0185, respectively). Again, all clinicopathological factors were not independent prognostic factors in terms of OS (Table 2) and LRPFS (Table 3).
Discussion
FDG-PET/CT image is now widely used as a routine pre-treatment staging work-up for many cancer types. In addition, it can provide more information for radiation oncologists in delineation of the target volumes. Recently, quantitative parameters of FDG-PET were also found to be a new kind of prognostic factor in various malignancies, including SCCHN (3,13,14,17,18).
Prior studies demonstrated that the SUVmax of the primary tumor was a significant prognostic factor for OS, DFS and local relapse-free survival in head and neck cancer (5-7,14). However, reports from other investigators could not support this finding (4,8-10,16). Results of the current study revealed that the SUVmax of the primary tumor did not correlate with OS and LRPFS. Although previous study showed nodal SUVmax was another significant prognostic factor for patients with oral cavity cancer treated by surgery (6), we did not use this parameter in our study because not all patients has detectable lymphadenopathies in the PET/CT image.
Recently, more and more studies demonstrated that MTV was a better prognostic predictor than the SUVmax of the primary tumor in OS, DFS and LRPFS (3,8-10,14,16,19). A review article by Castelli et al. showed that the MTV was well correlated to OS and DFS and had a higher predictive value than the SUVmax of the primary tumor (13). Another review article by Pak et al. also concluded that the MTV had significant predictive value in OS by pooled hazard ratio of 3.51 (2.62–4.72, P<0.00001) (14). Our study showed similar results that the MTV was a significant predictor in terms of OS and LRPFS by both univariate and multivariate analyses.
In our study, a higher proportion of patients with MTV ≥85 had clinical N2 disease (P=0.0728), which was correlated with clinical finding of increased tumor burden. Although most of our enrolled patients were T3–4 (92%), the MTV was not correlated with clinical T stage. The possible reason is the reflection of metabolic tumor burden by MTV instead of tumor size or invasion extent.
The new parameter of the TLG that reflects MTV plus FDG-uptake intensity of all lesions has been shown as a more significant predictor for treatment outcome in many malignancies. Several studies reported that the TLG was an important prognostic factor in predicting OS and DFS for head and neck cancer patients (14,17,18). A systematic review and meta-analysis by Pak et al. showed the TLG was a good predictor in OS by pooled hazard ratio of 3.14 (2.24–4.40, P<0.00001) (14). Our data support these findings. Besides, our study showed patients who had higher value of TLG had clinical T4 and N2 diseases. Although no significant difference was observed in TLG between clinical T and N stage, it was reasonable that more advanced disease represented more aggressive tumor behavior, which resulted in higher tumor metabolism.
The disadvantages of previous published literatures included (1) early to locally advanced diseases were all enrolled, which was a confounding factor due to its distinct prognosis, (2) various kinds of cancer sites were enrolled, which represented a much more heterogenous tumor behaviors, (3) inconsistent treatment modalities, which cause difficult judgment of treatment outcomes, and (4) shorter follow-up duration and could not well reflect the real impact of different FDG-PET quantitative parameters.
In our study, the primary local therapy for patients with oral cavity cancer is surgery. However, due to good tumor response of chemotherapy, some patients refused surgery and local RT became an alternative treatment options. For patients with oropharyngeal or hypopharyngeal cancer, organ preservation treatment with IndCT followed by RT/CCRT/Bio-RT.
The strength of this study is a uniform IndCT regimen followed by local therapy and adequate follow-up time compared with previous studies. Our results illustrate that the pretreatment MTV and TLG are two important independent prognostic factors for patients with advanced SCCHN patients. These findings should be verified by larger-scale prospective studies in the future.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (Available at https://tro.amegroups.com/article/view/10.21037/tro-02-5/coif). JCL serves as an unpaid editorial board member of Therapeutic Radiology and Oncology from Apr 2020 to Mar 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board in our institution (IRB TCVGH No: CE17010A). Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cancer Registry Annual Report, 2013, Health Promotion Administration, Ministry of Health and Welfare, Taiwan. Available online: https://www.hpa.gov.tw/File/Attach/5191/File_6166.pdf
- Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004;350:1945-52. [Crossref] [PubMed]
- Chung MK, Jeong HS, Park SG, et al. Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res 2009;15:5861-8. [Crossref] [PubMed]
- Allal AS, Dulguerov P, Allaoua M, et al. Standardized uptake value of 2-[18F]fluoro-2-deoxy-D-glucose in predicting outcome in head and neck carcinomas treated by radiotherapy with or without chemotherapy. J Clin Oncol 2002;20:1398-404. [Crossref] [PubMed]
- Liao CT, Chang JT, Wang HM, et al. Pretreatment primary tumor SUVmax measured by FDG-PET and pathologic tumor depth predict for poor outcomes in patients with oral cavity squamous cell carcinoma and pathologically positive lymph nodes. Int J Radiat Oncol Biol Phys 2009;73:764-71. [Crossref] [PubMed]
- Liao CT, Wang HM, Chang JT, et al. Influence of pathological nodal status and maximal standardized uptake value of the primary tumor and regional lymph nodes on treatment plans in patients with advanced oral cavity squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2010;77:421-9. [Crossref] [PubMed]
- Lin SC, Liao CY, Kao CH, et al. Pretreatment maximal standardized uptake value of the primary tumor predicts outcome to radiotherapy in patients with pharyngeal cancer. J Radiat Res 2012;53:462-8. [Crossref] [PubMed]
- Abgral R, Keromnes N, Robin P, et al. Prognostic value of volumetric parameters measured by 18FDG PET-CT in patients with head and neck squamous cell carcinoma. Eur J Nucl Med Mol Imaging 2014;41:659-67. [Crossref] [PubMed]
- Choi KH, Yoo LR, Han EJ, et al. Prognostic value of metabolic tumor volume measured by 18F-FDG PET/CT in locally advanced head and neck squamous cell carcinomas treated by surgery. Nucl Med Mol Imaging 2011;45:43-51. [Crossref] [PubMed]
- La TH, Fillion EJ, Turnbull BB, et al. Metabolic tumor volume predicts for recurrence and death in head and-neck cancer. Int J Radiat Oncol Biol Phys 2009;74:1335-41. [Crossref] [PubMed]
- Larson SM, Erdi Y, Akhurst T, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging: the visual response score and the change in total lesion glycolysis. Clin Positron Imaging 1999;2:159-71. [Crossref] [PubMed]
- Van de Wiele C, Kruse V, Smeets P, et al. Predictive and prognostic value of metabolic tumour volume and total lesion glycolysis in solid tumours. Eur J Nucl Med Mol Imaging 2013;40:290-301. [Crossref] [PubMed]
- Csatelli J, De Bari B, Depeursinge A, et al. Overview of the predictive value of quantitative 18 FDG PET in head and neck cancer treated with chemoradiotherapy. Crit Rev Oncol Hematol 2016;108:40-51. [Crossref] [PubMed]
- Pak K, Cheon GJ, Nam HY, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med 2014;55:884-90. [Crossref] [PubMed]
- Yu J, Cooley T, Truong MT, et al. Head and neck squamous cell cancer (stage III and IV) induction chemotherapy assessment: value of FDG volumetric imaging parameters. J Med Imaging Radiat Oncol 2014;58:18-24. [Crossref] [PubMed]
- Tang C, Murphy JD, Khong B, et al. Validation that metabolic tumor volume predicts outcome in head-and-neck cancer. Int J Radiat Oncol Biol Phys 2012;83:1514-20. [Crossref] [PubMed]
- Abd El-Hafez YG, Moustafa HM, Khalil HF, et al. Total lesion glycolysis: a possible new prognostic parameter in oral cavity squamous cell carcinoma. Oral Oncol 2013;49:261-8. [Crossref] [PubMed]
- Chang KP, Tsang NM, Liao CT, et al. Prognostic significance of 18F-FDG PET parameters and plasma Epstein-Barr virus DNA load in patients with nasopharyngeal carcinoma. J Nucl Med 2012;53:21-8. [Crossref] [PubMed]
- Higgins KA, Hoang JK, Roach MC, et al. Analysis of pretreatment FDG-PET SUV parameters in head-and neck cancer: tumor SUVmean has superior prognostic value. Int J Radiat Oncol Biol Phys 2012;82:548-53. [Crossref] [PubMed]
Cite this article as: Hsieh HY, Liu YC, Lin JW, Lin JC. Prognostic effects of the metabolic tumor volume and total lesion glycolysis in patients with advanced squamous cell carcinoma of head and neck. Ther Radiol Oncol 2018;2:5.


