Clinical outcome of head and neck adenoid cystic carcinoma in the Taiwan population: a single institution experience
Introduction
Adenoid cystic carcinoma (ACC) of head and neck is a relatively rare malignancy. It accounts for about 1% of all malignancies in the head and neck region and for about 22% in the major and minor salivary glands (1). ACC displays dual cell composition of peripheral myoepithelial and inner ductal tubular and cribriform patterns, and manifests indolent and variable biological behavior (2). Because of slow and insidious growth, ACC is often discovered at a late and locally advanced stage, especially in non-salivary gland area (3-5).
The main treatment for ACC is surgery. After surgery, adjuvant radiotherapy may confer a benefit on loco-regional control and survival in patients with high-risk pathological features, such as advanced T stage, close or positive margin, or perineural invasion (6-9). Nevertheless, relatively high rates of distant metastasis (especially to the lungs) pose a major obstacle in the management of ACC (6,10,11). Current published studies show low response rates and short response duration of chemotherapy and molecular therapies (12,13). One of the most important enzymes in ACC was c-kit protein, which was identified in 80–90% of the ACC tumor (14,15). Imatinib is a c-kit tyrosine kinase inhibitor that is used in cancer treatment. Several studies researched the benefit of imatinib in ACC, but no obvious response was noted in advanced ACC (16,17).
Because of the indolent nature of ACC, improving local control and reducing distant metastasis control may warrant different treatment strategies. Prognosis may also differ depending on the site of primary origin. Therefore, based on the evidence we currently have, we aimed to explore the clinical features and prognosis of ACC in the head and neck region in our institution.
Methods
Patients and work-up
We collected patients diagnosed as adenoid cystic carcinoma (ACC) in the head and neck region between January 1995 and December 2014 from the database of National Cheng Kung University Hospital. We retrospectively reviewed medical records of these patients. Patients who were diagnosed as head and neck ACC and received treatment under curative intent were included in this study. We excluded patients who had previous irradiation to head and neck region, severe systemic disease (e.g., history of cirrhosis, heart failure, coronary artery disease, or stroke), previous malignancy, planned radiation dose under 50 Gy, and patients with distant metastasis at the time of diagnosis.
The pretreatment workup included general physical examination, chest X-ray, abdominal sonography, head and neck computed tomography, and completed blood count/biochemical profiles. Patient data including age, gender, operation notes, histology, margin status, perineural invasion, radiotherapy, chemotherapy, and outcome variables were collected from the hospital records of each patient. The study obtained approval from the Institutional Review Board (IRB) of National Cheng Kung University Hospital (IRB number: A-ER-104-279).
Treatment
All patients received curative surgery except for three, who were treated with primary concurrent chemoradiotherapy. Adjuvant radiotherapy was suggested for patients with adverse pathological features, such as T3-4, close/positive surgical margin, perineural invasion, positive lymph node involvement, or inadequate surgical excision.
Because these patients were treated from 1995 to 2014, RT technique was divided into two major categories. Before 2006, conventional 2-dimensional or 3-dimensional (2D/3D) RT was the major RT technique for these patients. In 2D/3D RT era, elective nodal irradiation was performed according to the clinician’s judgement. After 2006, inverse planning software and intensity-modulated radiation therapy were used. The high-risk clinical target volume (CTV) was prescribed to 66–70 Gy, which covered the primary tumor, surgical tumor bed, area where soft tissue was invaded, the risk area along the cranial nerve, and extracapsular spread (ECS). The intermediate-risk CTV was prescribed to 59.4–64.8 Gy, which included the entire surgical bed and the involved neck levels. The low-risk CTV was prescribed at 46–50 Gy and included uninvolved neck levels at risk for occult micrometastases. Radiotherapy was given in conventional fractionation, 1.8–2 Gy per fraction for 5 consecutive days per week. Cisplatin was the most frequently used concurrent chemotherapy regimen, with 100 mg/m2 delivered once every 3 weeks or 40 mg/m2 delivered once per week.
Patients follow-up and end-points
Patients were followed every 1 to 3 months in the first year, every 3–6 months in the second and third year, and 6–12 months in the following years after completion of treatment. Comprehensive physical examination was performed during each visit. Imaging studies such as computed tomography (CT) or magnetic resonance imaging (MRI) and chest X-ray were arranged 2 to 3 months after end of radiotherapy, and then once annually or as clinically indicated.
Overall survival (OS) was defined from the date of definitive treatment (surgery or definitive radiotherapy in unresectable disease) to death from any cause. Loco-regional recurrence-free survival (LRFS) was the duration between the definitive treatment to loco-regional recurrence. Distant metastasis-free survival (DMFS) was the duration between definitive treatment to distant metastasis. Disease free survival (DFS) was defined as the time from definitive treatment to the date of loco-regional recurrence, distant metastasis, or secondary cancer.
Statistical analysis
The differences among clinical or pathological features were calculated by Pearson chi-squared test, Fisher’s exact test, Mann-Whitney U-test, or independent t-test as appropriate. Survival curves were calculated and plotted with the Kaplan-Meier method and log-rank test. We assumed variables, such as gender, age, tumor site, T stage, nodal status, surgery, margin, perineural invasion, radiotherapy, and chemotherapy, and used the stepwise Cox regression model for multivariate analysis. Commercial statistical software (SPSS 17.0; IBM Corporation, Armonk, New York, USA) was used for all analyses.
Results
Patient characteristics
In total, 53 patients were included in this study. Baseline patient characteristics are listed in Table 1. The mean patient age was 52.66 years (range, 23–92 years) and the median follow-up time was 5.65 years (range, 1.89–19.48 years). More patients with salivary gland origin were diagnosed with early stage when compared to patients with non-salivary gland origin (P=0.019). In the non-salivary gland group, there were 5 patients with oral origin, 12 patients with nasal and paranasal sinus origin, 2 patients with oropharynx origin, and 1 patient with nasopharynx origin. Most patients received definitive surgery, but 3 patients in the non-salivary gland group received definitive chemo-radiotherapy (reasons were un-resectable disease, nasopharyngeal primary site, and patient refusal). Among patients receiving definitive surgery, neck lymph node dissection was performed in 11 patients. The percentage of patients receiving radiotherapy (P=0.095) or chemotherapy (P=0.272) was similar in patients with both salivary gland origin and non-salivary gland origins. Thirty-five patients received radiotherapy (median dose, 66 Gy; range, 55–72 Gy), of which 9 received chemoradiotherapy. Concurrent chemotherapy was cisplatin-based except for 2 patients who had concurrent oral Uracil-Tegafur (UFUR).
Table 1
| Patient characteristics | Salivary gland (n=33) | Non-salivary gland (n=20) | P value |
|---|---|---|---|
| Gender (male) | 18 (54.5) | 11 (55.0) | 0.974 |
| Mean age (year) | 51.33±14.56 | 54.85±12.36 | 0.372 |
| Cigarette | 6 (18.2) | 5 (25.0) | 0.804 |
| Alcohol | 4 (12.1) | 2 (10.0) | 0.779 |
| Primary tumor stagea | 0.019 | ||
| T1 | 9 (27.3) | 6 (30.0) | |
| T2 | 16 (48.5) | 1 (5.0) | |
| T3 | 8 (24.2) | 5 (25.0) | |
| T4 | 0 (0) | 8 (40.0) | |
| Nodal statusa | 0.138 | ||
| Positive neck LN | 0 (0) | 2 (10.0) | |
| Surgery | 33 (100.0) | 17 (85.0) | 0.049 |
| Closeb/positive margin | 15 (45.5) | 7 (35.0) | 0.703 |
| Perineural invasion | 16 (48.5) | 4 (20.0) | 0.073 |
| Radiotherapyc | 19 (57.6) | 16 (80.0) | 0.095 |
| Chemotherapyc | 4 (12.1) | 5 (25.0) | 0.272 |
a, initial clinical status; b, ≤1 mm; c, only receive definitive and/or adjuvant aim management was included.
Survival
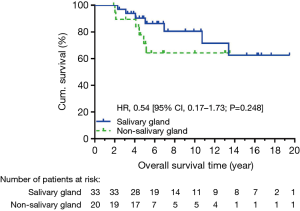
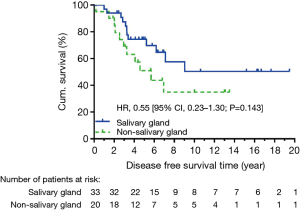
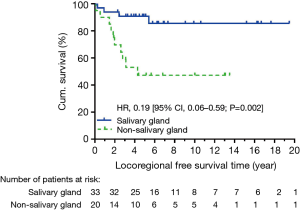
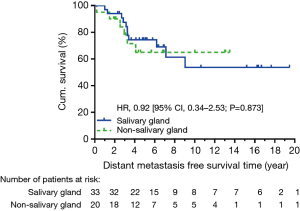
At data cut-off in December 2015, 40 patients were alive, and we observed 14 locoregional recurrent events and 17 distant metastatic events. The causes of death were ACC in 10 patients, secondary cancer in 2 patients, and non-cancer event in 1 patient. Five-year OS was not significantly different between salivary and non-salivary gland ACC (90.4% vs. 71.4%, HR: 0.54, CI: 0.17–1.73, P=0.248) (Figure 1). There was also no significant difference in 5-year DFS (HR: 0.55, CI: 0.23–1.30, P=0.143) (Figure 2). None of the locoregional recurrent events (N=14) occurred in regional lymph nodes. Fourteen patients suffered local-regional recurrence during follow up, and all were primary recurrence. Nine of them received adjuvant radiotherapy, and all recurrence was in-field. Patients with non-salivary gland origin had worse locoregional recurrence-free survival than those with salivary gland origin (HR: 0.19, CI: 0.06–0.59, P=0.002) (Figure 3). All 17 distant metastatic sites were the lungs and the median time to metastasis was 3.14 years (range, 0.18–9.04 years). DMFS was similar in the two groups (HR: 0.92, CI: 0.34–2.53, P=0.873) (Figure 4).
In patients who received definitive surgery, adjuvant radiotherapy showed a trend towards improving 5-year locoregional recurrence-free survival (80.9% vs. 71.4%, P=0.616). Among the 3 patients who received primary CCRT, we observed 2 local recurrences and 2 distant metastases.
Prognostic factors
In univariate analysis (Table 2), non-salivary gland origin (HR: 5.19, CI: 1.62–16.60, P=0.006) and advanced T stage (HR: 5.56, CI: 1.72–18.03, P=0.004) significantly impacted locoregional control, while a trend was observed in nodal positive patients (HR: 4.52, CI: 1.00–20.47, P=0.050). However, in the multivariate analysis (Table 3), female gender, non-salivary site, nodal positive, close/positive margin, and adjuvant radiotherapy all correlated with locoregional recurrence-free survival.
Table 2
| Variable | HR | 95% CI of HR | P value |
|---|---|---|---|
| Female gender | 2.45 | 0.82–7.32 | 0.109 |
| Age (in year) | 1.01 | 0.97–1.04 | 0.687 |
| Non-salivary site | 5.19 | 1.62–16.60 | 0.006 |
| T3/4 stagea | 5.56 | 1.72–18.03 | 0.004 |
| Nodal positive | 4.52 | 1.00–20.47 | 0.050 |
| Surgery | 0.39 | 0.09–1.74 | 0.214 |
| Close/positive margin | 1.26 | 0.41–3.93 | 0.685 |
| Perineural invasion | 0.46 | 0.12–1.68 | 0.238 |
| Radiotherapy | 0.88 | 0.30–2.64 | 0.824 |
| Chemotherapy | 1.41 | 0.39–5.09 | 0.596 |
a, reference category: T1/2 stage. HR, hazard ratio; CI, confidence interval.
Table 3
| Variable | HR | 95% CI of HR | P value |
|---|---|---|---|
| Female gender | 18.35 | 2.10–160.67 | 0.009 |
| Age (in year) | 1.02 | 0.97–1.08 | 0.377 |
| Non-salivary site | 19.26 | 2.67–138.00 | 0.003 |
| T3/4 stagea | 5.41 | 0.87–33.52 | 0.070 |
| Nodal positive | 87.69 | 1.92–4,041.49 | 0.022 |
| Close/positive margin | 25.02 | 2.11–297.50 | 0.011 |
| Perineural invasion | 0.35 | 0.04–3.15 | 0.350 |
| Radiotherapy | 0.01 | 0.000–0.20 | 0.003 |
| Chemotherapy | 0.57 | 0.04–8.41 | 0.679 |
a, reference category: T1/2 stage. HR, hazard ratio; CI, confidence interval.
Discussion
ACC is a relatively rare disease in the head and neck region (18). In a period of 19 years, only 53 patients seeking treatment in our institution had this cancer. We found that non-salivary gland ACC had worse locoregional control rate than salivary gland ACC, but no difference was demonstrated in DFS or OS. After surgery, adjuvant radiotherapy conferred benefit on locoregional control. Limited neck lymph node involvement was noted in initial status and no neck nodal recurrence was detected in our series.
Our results are in line with contemporary reports, with an overall 5-year survival outcome for ACC in all head and neck subsites of 83.2%. Ellington et al. reported 90.3%, 79.9%, and 69.2% in 5-year, 10-year, and 20-year relative survival rates for all head and neck sites (19), while Dubal et al. reported 85.9%, 75.6%, and 53.5% survival rates for oral cavity ACC (20). Moreover, studies have indicated that ACC of nasal cavity and paranasal sinus origin had a poor prognosis, often characterized by late diagnosis with an advanced tumor stage (T3/4) (4,5). In our study, a more advanced tumor stage (65% vs. 24.2%) was also observed in the non-salivary gland group.
In our study, although adjuvant radiotherapy failed to show a statistically significant benefit on locoregional control on univariate analysis, a locoregional control benefit was found on multivariable analysis. Previous studies also suggested surgery combined with postoperative radiotherapy reduces local recurrence and improves disease-free survival (6,7,9). In conditions such as incomplete tumor resection, advanced stage, solid pattern, poorly differentiated histology, or cervical lymph node metastasis, the benefit of adjuvant radiotherapy may be more certain (21,22). Postoperative radiotherapy combined with chemotherapy may improve locoregional control, but not OS (23,24). However, we did not find a benefit in locoregional control with chemotherapy in our study. Currently, the chemotherapy agents tested had limited response rate and no standard regimen could be recommended (13).
In our series, only 2 of 53 patients had initial clinical nodal metastasis, and no patient experienced a nodal recurrence. Suárez et al. showed the variable incidence of cervical nodal metastasis in head and neck ACC (HNACC), which ranged between 3-16%, while the incidence of nodal recurrence is 0-14% after treatment (25). However, there is currently no consensus on the benefit of elective neck dissection (END) in HNACC. Lee et al. showed worse OS in nodal-positive patients and recommended END for regional control (26). Amit et al. reported higher nodal involvement (37%) in oral cavity ACC, and suggested END in these patients (27). However, several studies showed limited survival benefits of routine END (25,28,29), and recommended END should only be considered in selective patients with higher positive nodal ratio (30) or high risk oral and oropharyngeal locations, especially when postoperative radiotherapy was not planned (25).
On multivariable analysis, we also found factors contributing to worse locoregional control, such as female, close/positive margins, and no adjuvant radiotherapy. Several retrospective studies indicated ACC was predominant in female gender (20,31,32). However, Ellington et al. (19) indicated female patients were found to have significantly better survival rate across all time periods, which conflicted with our results. Past literature had confirmed resection with clear margins was the gold standard management of these patients (21,22,33,34). In our study, perineural invasion was not a prognostic factor in local control. However, the incidence of perineural invasion ranged from 29.4% to 62.5% (32) and was an independent prognostic factor in salivary gland ACC in many studies (35-37). A meta-analysis of ACC of the nasal cavity and paranasal sinuses showed that tumor margin and site were also major prognostic factors, whereas perineural invasion was not (5). Although perineural invasion had no survival impact, intraneural invasion may be a prognostic factor in HNACC (38).
There were several limitations in our study. First, this study is retrospective in nature, thus management strategies are prone to patient selection. However, we collected consecutive HNACC patients under curative intent treatment in our institution, which adequately represents clinical practice. Second, our study only included patients who were diagnosed in our institution. Due to rarity of this disease, patient numbers may be inadequate to draw definite conclusions. Third, treatment techniques (3D-confromal radiotherapy or IMRT) may differ as the cohort of patients spanned during a long period of time. Surgical techniques, pathology consensus, and treatment guidelines have evolved during this period. In conclusion, non-salivary gland ACC has a worse loco-regional control rate than salivary gland ACC. Adjuvant radiotherapy confers a benefit on loco-regional control, but the benefit of elective nodal irradiation could not be evaluated in this study. In these patients, the most common pattern of failure was distant metastasis, especially to the lungs. No benefit form cisplatin-based chemotherapy was found. Further research is warranted to improve outcomes in these patients.
Acknowledgments
We thank Clare Lear for editing this manuscript.
Funding: This work was supported by National Cheng Kung University Hospital, Tainan (grant number NCKUH-10503017).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study obtained approval from the Institutional Review Board (IRB) of National Cheng Kung University Hospital (IRB number: A-ER-104-279). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kokemueller H, Eckardt A, Brachvogel P, et al. Adenoid cystic carcinoma of the head and neck--a 20 years experience. Int J Oral Maxillofac Surg 2004;33:25-31. [Crossref] [PubMed]
- Li J, Perlaky L, Rao P, et al. Development and characterization of salivary adenoid cystic carcinoma cell line. Oral Oncol 2014;50:991-9. [Crossref] [PubMed]
- Andrade MF, de Faria PR, Cardoso SV, et al. Adenoid cystic carcinoma of the maxillary sinus: a clinical-pathological report of 10 years of experience from a single institution. Int J Oral Maxillofac Surg 2014;43:1313-8. [Crossref] [PubMed]
- Michel G, Joubert M, Delemazure AS, et al. Adenoid cystic carcinoma of the paranasal sinuses: retrospective series and review of the literature. Eur Ann Otorhinolaryngol Head Neck Dis 2013;130:257-62. [Crossref] [PubMed]
- Amit M, Binenbaum Y, Sharma K, et al. Adenoid cystic carcinoma of the nasal cavity and paranasal sinuses: a meta-analysis. J Neurol Surg B Skull Base 2013;74:118-25. [Crossref] [PubMed]
- Shen C, Xu T, Huang C, et al. Treatment outcomes and prognostic features in adenoid cystic carcinoma originated from the head and neck. Oral Oncol 2012;48:445-9. [Crossref] [PubMed]
- Balamucki CJ, Amdur RJ, Werning JW, et al. Adenoid cystic carcinoma of the head and neck. Am J Otolaryngol 2012;33:510-8. [Crossref] [PubMed]
- Silverman DA, Carlson TP, Khuntia D, et al. Role for Postoperative Radiation Therapy in Adenoid Cystic Carcinoma of the Head and Neck. The Laryngoscope 2004;114:1194-9. [Crossref] [PubMed]
- Mendenhall WM, Morris CG, Amdur RJ, et al. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck 2004;26:154-62. [Crossref] [PubMed]
- Bhayani MK, Yener M, El-Naggar A, et al. Prognosis and risk factors for early-stage adenoid cystic carcinoma of the major salivary glands. Cancer 2012;118:2872-8. [Crossref] [PubMed]
- Sung MW, Kim KH, Kim JW, et al. Clinicopathologic predictors and impact of distant metastasis from adenoid cystic carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg 2003;129:1193-7. [Crossref] [PubMed]
- Dodd RL, Slevin NJ. Salivary gland adenoid cystic carcinoma: a review of chemotherapy and molecular therapies. Oral Oncol 2006;42:759-69. [Crossref] [PubMed]
- Papaspyrou G, Hoch S, Rinaldo A, et al. Chemotherapy and targeted therapy in adenoid cystic carcinoma of the head and neck: a review. Head Neck 2011;33:905-11. [Crossref] [PubMed]
- Jeng YM, Lin CY, Hsu HC. Expression of the c-kit protein is associated with certain subtypes of salivary gland carcinoma. Cancer Lett 2000;154:107-11. [Crossref] [PubMed]
- Holst VA, Marshall CE, Moskaluk CA, et al. KIT protein expression and analysis of c-kit gene mutation in adenoid cystic carcinoma. Mod Pathol 1999;12:956-60. [PubMed]
- Hotte SJ, Winquist EW, Lamont E, et al. Imatinib mesylate in patients with adenoid cystic cancers of the salivary glands expressing c-kit: a Princess Margaret Hospital phase II consortium study. J Clin Oncol 2005;23:585-90. [Crossref] [PubMed]
- Pfeffer MR, Talmi Y, Catane R, et al. A phase II study of Imatinib for advanced adenoid cystic carcinoma of head and neck salivary glands. Oral Oncol 2007;43:33-6. [Crossref] [PubMed]
- Bjørndal K, Krogdahl A, Therkildsen MH, et al. Salivary gland carcinoma in Denmark 1990-2005: a national study of incidence, site and histology. Results of the Danish Head and Neck Cancer Group (DAHANCA). Oral Oncol 2011;47:677-82. [Crossref] [PubMed]
- Ellington CL, Goodman M, Kono SA, et al. Adenoid cystic carcinoma of the head and neck: Incidence and survival trends based on 1973-2007 Surveillance, Epidemiology, and End Results data. Cancer 2012;118:4444-51. [Crossref] [PubMed]
- Dubal PM, Unsal AA, Chung SY, et al. Population-based trends in outcomes in adenoid cystic carcinoma of the oral cavity. Am J Otolaryngol 2016;37:398-406. [Crossref] [PubMed]
- Li Q, Zhang XR, Liu XK, et al. Long-term treatment outcome of minor salivary gland carcinoma of the hard palate. Oral Oncol 2012;48:456-62. [Crossref] [PubMed]
- Bjørndal K, Krogdahl A, Therkildsen MH, et al. Salivary adenoid cystic carcinoma in Denmark 1990-2005: Outcome and independent prognostic factors including the benefit of radiotherapy. Results of the Danish Head and Neck Cancer Group (DAHANCA). Oral Oncol 2015;51:1138-42. [Crossref] [PubMed]
- Schoenfeld JD, Sher DJ, Norris CM Jr, et al. Salivary gland tumors treated with adjuvant intensity-modulated radiotherapy with or without concurrent chemotherapy. Int J Radiat Oncol Biol Phys 2012;82:308-14. [Crossref] [PubMed]
- Hsieh CE, Lin CY, Lee LY, et al. Adding concurrent chemotherapy to postoperative radiotherapy improves locoregional control but Not overall survival in patients with salivary gland adenoid cystic carcinoma-a propensity score matched study. Radiat Oncol 2016;11:47. [Crossref] [PubMed]
- Suárez C, Barnes L, Silver CE, et al. Cervical lymph node metastasis in adenoid cystic carcinoma of oral cavity and oropharynx: A collective international review. Auris Nasus Larynx 2016;43:477-84. [Crossref] [PubMed]
- Lee SY, Kim BH, Choi EC. Nineteen-year oncologic outcomes and the benefit of elective neck dissection in salivary gland adenoid cystic carcinoma. Head Neck 2014;36:1796-801. [Crossref] [PubMed]
- Amit M, Binenbaum Y, Sharma K, et al. Incidence of cervical lymph node metastasis and its association with outcomes in patients with adenoid cystic carcinoma. An international collaborative study. Head Neck 2015;37:1032-7. [Crossref] [PubMed]
- Amit M, Na'ara S, Sharma K, et al. Elective neck dissection in patients with head and neck adenoid cystic carcinoma: an international collaborative study. Ann Surg Oncol 2015;22:1353-9. [Crossref] [PubMed]
- Coca-Pelaz A, Barnes L, Rinaldo A, et al. Cervical Lymph Node Metastasis in Adenoid Cystic Carcinoma of the Larynx: A Collective International Review. Adv Ther 2016;33:553-79. [Crossref] [PubMed]
- Liu Z, Fang Z, Dai T, et al. Higher positive lymph node ratio indicates poorer distant metastasis-free survival in adenoid cystic carcinoma patients with nodal involvement. J Craniomaxillofac Surg 2015;43:751-7. [Crossref] [PubMed]
- Jaafari-Ashkavandi Z, Ashraf MJ, Moshaverinia M. Salivary gland tumors: a clinicopathologic study of 366 cases in southern Iran. Asian Pac J Cancer Prev 2013;14:27-30. [Crossref] [PubMed]
- Dantas AN, Morais EF, Macedo RA, et al. Clinicopathological characteristics and perineural invasion in adenoid cystic carcinoma: a systematic review. Braz J Otorhinolaryngol 2015;81:329-35. [Crossref] [PubMed]
- Lukšić I, Suton P, Macan D, et al. Intraoral adenoid cystic carcinoma: is the presence of perineural invasion associated with the size of the primary tumour, local extension, surgical margins, distant metastases, and outcome? Br J Oral Maxillofac Surg 2014;52:214-8. [Crossref] [PubMed]
- Meyers M, Granger B, Herman P, et al. Head and neck adenoid cystic carcinoma: A prospective multicenter REFCOR study of 95 cases. Eur Ann Otorhinolaryngol Head Neck Dis 2016;133:13-7. [Crossref] [PubMed]
- Ge MH, Wang JF, Xia QM, et al. Prognostic analysis of 76 cases with adenoid cystic carcinoma in salivary gland. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012;47:202-6. [PubMed]
- Wang JF, Ge MH, Wang KJ, et al. Clinical analysis of 52 cases of adenoid cystic carcinoma in minor salivary gland. Zhonghua Kou Qiang Yi Xue Za Zhi 2012;47:705-10. [PubMed]
- Ju J, Li Y, Chai J, et al. The role of perineural invasion on head and neck adenoid cystic carcinoma prognosis: a systematic review and meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol 2016;122:691-701. [Crossref] [PubMed]
- Amit M, Binenbaum Y, Trejo–Leider L, et al. International collaborative validation of intraneural invasion as a prognostic marker in adenoid cystic carcinoma of the head and neck. Head Neck 2015;37:1038-45. [Crossref] [PubMed]
Cite this article as: Chan KH, Tsai MH, Cheng YJ, Wu YH. Clinical outcome of head and neck adenoid cystic carcinoma in the Taiwan population: a single institution experience. Ther Radiol Oncol 2018;2:4.