Salvage radiotherapy for biochemical recurrence after radical prostatectomy: experience of a single center
Introduction
Prostate cancer was the fifth most diagnosed cancer and the seventh most common cause of cancer death among males in Taiwan with a crude incidence of 41 per 100,000 (1). Radical prostatectomy (RP) has been one of the standard definitive treatment for localized prostate cancer. However, many studies showed that around 25–40% of patients develop biochemical recurrence (BCR) following RP for localized prostate cancer (2,3). Salvage radiotherapy (SRT) for BCR after RP has been reported to improve outcomes (4-8). Therefore, SRT is one of the standard treatment for BCR following RP (9-11). However, even patients received SRT, the 5-year biochemical disease-free survival rates remained 50–60%. The identification of useful predictors is very important to select patients at high risk of BCR after RP. Recent retrospective studies have reported various prognostic factors related to PSA failure after SRT (6,12-18). The prognostic factors included the pathologic Gleason score, surgical margin status, seminal vesicle invasion, pre-RT PSA level, PSA doubling time.
Most of the studies of SRT following RP are reported by westerner. There were few studies from Asia (19-21). Thus, we investigated the outcome and prognostic factors of patients who received SRT for BCR after RP and whether concurrent with androgen deprivation therapy (ADT) could improve outcomes at a single center.
Methods
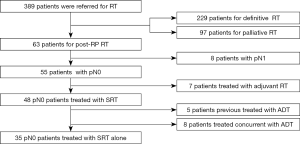
In our institution, 63 patients underwent RP with pelvic lymph node dissection and post-operative RT for prostate cancer between January 2004 and December 2012 (Figure 1). Of 63 patients, 8 patients who were pN1 and 7 patients who received adjuvant RT were excluded from the study. The remaining 48 patients were included in this study. Among them, 5 patients were previously treated with ADT and 8 patients were treated concurrently with ADT. All patients had done systemic survey and showed no distant metastasis before they received SRT. We reviewed the medical records of the 48 patients receiving SRT for BCR after RP.
Tumors were classified according to the 7th, ED, 2010 AJCC staging system (22). Adjuvant RT was defined as immediate RT given within 6 months after RT with an undetectable PSA (<0.2 ng/mL) (23). The definition of SRT was patients receiving RT on post-RP serum PSA failure (at least two consecutive PSA elevations ≥0.2ng/mL) or persistent PSA after RP. SRT was delivered with intensity modulation radiotherapy (IMRT) technique and was prescribed at a total dose of 60–75.6 Gy (median 64 Gy) with a daily dose of 1.8–2.0 Gy, 5 days per week. The clinical target volume (CTV) was defined as adequately covering prostatic fossa. Elective pelvic lymphatics irradiation for 45 Gy was delivered in 5 patients (10.4% of the total group). The elective pelvic lymphatics irradiation was judged by individual physicians. Written informed consent was obtained from all patients before the start of SRT, and patients were informed of both the benefits and complications of SRT.
After RP and SRT, prostate-specific antigen (PSA) levels were followed up every 3–6 months during the first 5 years, then at least once per year. BCR after SRT was defined as the Phoenix Definition, a rise by ≥2 ng/mL above the nadir PSA. Early SRT was defined as started RT before the PSA level >0.5 ng/mL. Acute toxicities associated to RT was recorded according to the Common Terminology Criteria for Adverse Events version 3.0.
We analyzed clinic-pathologic factors including age, performance, initial PSA levels, pathologic stages, surgical margin statuses, Gleason scores of surgical specimen, seminal vesicle invasion, post-RP PSA nadir, pre-RT PSA levels, RT dose, RT field, and previously treated with or concurrent with ADT. We analyzed whether SRT combining with ADT improved the outcomes.
The effects of different factors on the biochemical failure following SRT were analyzed using univariate and multivariate Cox proportional hazard regression models. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated. BCR-free survival rates, clinical progression-free survival rates (local-regional relapse and distant metastasis), and overall survival rates were assessing via Kaplan–Meier method and the log-rank test. Probability values of <0.05 were considered statistically significant. All statistical analyses were performed using a commercial software (IBM SPSS version 22.0, Armonk, NY, USA). This study was approved by the Institutional Review Board of China Medical University Hospital (CMUH106-REC1-060).
Results
The patients’ clinical and pathological characteristics are shown in Table 1. The median age at the initiation of RT was 69 years old (range, 54–84 years). The median post-RP PSA nadir was 0.109 ng/mL (range, <0.003–6.3 ng/mL). The median pre-RT PSA level was 0.483 ng/mL (range, 0.159–4.912 ng/mL). The median PSA doubling time was 5.5 months (range, 1–60 months). The median RT dose was 64 Gy (range, 60–75.6 Gy). The median interval from RP to SRT was 23.8 months (range, 2.2–181.5 months).
Table 1
| Factors | Categorization | SRT (%), n=48 |
|---|---|---|
| Age (years) | <70 | 25 (52.1) |
| ≥70 | 23 (47.9) | |
| Initial PSA (ng/mL) | <10 | 16 (36.4) |
| 10–20 | 14 (31.8) | |
| ≥20 | 14 (31.8) | |
| Gleason score | ≤6 | 11 (24.4) |
| 7 | 22 (48.9) | |
| 8–10 | 12 (26.7) | |
| Pathologic T stage | 2 | 18 (38.3) |
| 3 | 28 (59.6) | |
| 4 | 1 (2.1) | |
| Extracapsular extension | − | 11 (26.8) |
| + | 30 (73.2) | |
| Margin | − | 21 (48.8) |
| + | 22 (51.2) | |
| Seminal vesicle invasion | − | 28 (63.6) |
| + | 16 (36.4) | |
| Post-RP PSA nadir (ng/mL) | ≤0.1 | 23 (52.3) |
| >0.1 | 21 (47.7) | |
| RP to RT interval (months) | ≤24 | 24 (50.0) |
| >24 | 24 (50.0) | |
| ADT with RT | − | 35 (72.9) |
| + | 13 (27.1) | |
| Pre-RT PSA (ng/mL) | ≤0.5 | 25 (52.1) |
| >0.5 | 23 (47.9) | |
| PSA doubling time (months) | <5 | 18 (42.9) |
| ≥5 | 24 (57.1) | |
| RT dose (cGy) | 6,000–6,400 | 28 (58.3) |
| 6,600–6,840 | 12 (25.0) | |
| 7,000–7,560 | 8 (16.7) | |
| RT field | Prostate fossa | 43 (89.6) |
| pelvis | 5 (10.4) |
SRT, salvage radiotherapy; BCR, biochemical recurrence; RP, radical prostatectomy; ADT, androgen deprivation therapy.
With a median follow up of 68.7 months (range, 34.0–143.3 months) after SRT, 16 patients (33.3%) experienced BCR. Eight patients (16.7%) developed clinical recurrence. Among them, 1 patient subsequently developed local-regional relapse and distant metastasis, 1 had only local relapse, 2 had both pelvic LN relapse and distant metastasis, and 4 developed distant metastasis only. All clinical recurrent patients had BCR first.
The BCR-free survival rates at 3 and 5 years for the 48 patients after SRT were 72.9% and 68.4%, respectively (Figure 2A). The median interval from SRT to BCR was 4.86 years (range, 0.34–10.13 years). The clinical progression-free survival rates at 3 and 5 years were 89.6% and 86.6%, respectively (Figure 2B). At the time of final analysis, 6 (12.5%) of 48 patients had died. Five-year overall survival was 92.7% (Figure 2C). Cancer progression related death was noted in three patients. The three other deaths were due to AML, heart failure, and in one case, unknown causes. No treatment-related deaths occurred.
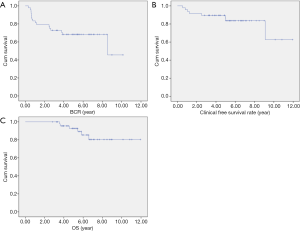
Table 2 shows the Cox proportional hazard regression analyses of the different prognostic factors thought to contribute to BCR-free survival. Univariate analysis showed pre-RT PSA level >0.5 ng/mL, Gleason score at RP ≥8, and seminal vesicle invasion were significant predictive parameters for PSA progression after SRT (P=0.021, 0.006, and 0.024). Multivariate analysis revealed similar result to that of univariate analysis.
Table 2
| Prognostic factor | Categorization | Univariate | Multivariable | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |||
| Age (years) | <70 | 1 | 0.853 | – | – | |
| ≥70 | 1.124 (0.325–3.884) | |||||
| Initial PSA (ng/mL) | <20 | 1 | 0.756 | 1 | 0.111 | |
| ≥20 | 1.239 (0.32–4.794) | 5.304 (0.683-41.173) | ||||
| Pathologic T stage | ≤2 | 1 | 0.369 | 1 | 0091 | |
| ≥3 | 2.035 (0.432–9.588) | 0.042 (0.001–1.654) | ||||
| Gleason score | ≤7 | 1 | 0.006 | 1 | 0.018 | |
| 8–10 | 6.045 (1.695–21.563) | 7.919 (1.424–44.022) | ||||
| Extracapsular extension | − | 1 | 0.369 | 1 | 0.166 | |
| + | 2.035 (0.432–9.588) | 8.866 (0.404–194.757) | ||||
| Seminal vesicle invasion | − | 1 | 0.024 | 1 | 0.005 | |
| + | 4.319 (1.213–15.385) | 21.731(2.510–188.177) | ||||
| Surgical margin | − | 1 | 0.509 | – | – | |
| + | 0.652 (0.183–2.319) | |||||
| Post-RP PSA nadir (ng/ml) | ≤0.1 | 1 | 0.204 | 1 | 0.185 | |
| >0.1 | 2.272 (0.64–8.068 | 0.260 (0.35–1.908) | ||||
| RT dose (cGy) | ≤6400 | 1 | 0.706 | – | – | |
| ≥6600 | 1.276 (0.36–4.523) | |||||
| Pre-RT PSA (ng/mL) | ≤0.5 | 1 | 0.021 | 1 | 0.035 | |
| >0.5 | 6.249 (1.322–29.531) | 23.294(1.249–434.410) | ||||
| RT field | Prostate fossa | 1 | 0.347 | – | – | |
| pelvis | 0.040 (0–32.358) | |||||
| PSA doubling time (months) | <5 | 1 | 0.553 | 1 | 0.400 | |
| ≥5 | 1.456 (0.421–5.034) | 2.080 (0.377–11.465) | ||||
| ADT | − | 1 | 0.182 | 1 | 0.525 | |
| + | 2.367 (0.667–8.397) | 1.939 (0.252–14.952) | ||||
RT, radiotherapy; ADT, androgen deprivation therapy.
Figure 3A-C shows BCR-free survival rates using the Kaplan-Meier method according to the pre-RT PSA level, Gleason score, and seminal vesicle invasion. Among the 48 patients, early SRT was administered to 25 patients (52%). In this subgroup, 5-year BCR-free survival rate was 83.4% in the pre-RT PSA level ≤0.5 ng/mL group and 52.2% in the pre-RT PSA level >0.5 ng/mL group (P=0.007, log-rank test). Twelve of 48 (27%) patients had Gleason score ≥8. Five-year BCR-free survival rate was 78.4% in the Gleason score <8 group and 41.7% in the Gleason score ≥8 group (P=0.008, log-rank test). Sixteen of 48 (36%) patients had seminal vesicle invasion. Five-year BCR-free survival rate was 75.2% in the seminal vesicle free group and 52.3% in the seminal vesicle invasive group (P=0.064, log-rank test).

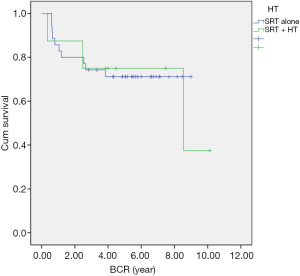
Comparing SRT alone with SRT concurrent with ADT, the 5-year BCR-free survival rate was 71.2% in the RT alone group and 75% in the RT concurrent with ADT group (P=0.928; Figure 4).
Grade 2 acute genitourinary adverse events were noted in 2 (4.2%) of 48 patients. Grade 2 acute gastrointestinal adverse events were noted in 4 (8.3%) of 48 patients. No grade 3 or worse acute adverse events were noted.
Discussion
In the current study, the 3- and 5-year BCR-free survival rate after SRT were 72.9% and 68.4%, respectively. The rates were compatible with other studies, ranging from 40% to 70% (6,12,13,16-21). The 5-year clinical progression-free survival rate and distant metastasis rate after SRT were 86.6% and 88.6%, respectively.
Several prognostic factors for SRT have been found, such as pre-RT PSA levels, Gleason scores, seminal vesicle invasion, surgical margin status, and PSA doubling time (13,17). A large systemic review of 41 retrospective studies with 5,597 patients on SRT following RP illustrated that the pre-RT PSA levels was significantly associated with BCR-free survival (24). There was an average of 2.6% loss of BCR- free survival for each incremental 0.1 ng/mL of PSA at the time of SRT. Although current guidelines only recommend a pre-RT PSA <1.0 ng/mL (9,11), several studies have reported that patients who received SRT at pre-RT PSA ≤0.5 ng/mL had better outcomes (4,6,19,25-27). There were no clear definitions for PSA persistence and PSA recurrence. Consensus has not defined a threshold level of PSA below which it is truly undetectable. Retrospective studies reported patients with undetectable post-RP PSA had better disease-free survival, the threshold levels were 0.05 and 0.01 ng/mL, respectively. Recent data from Taiwan reported a post-RP PSA nadir ≤0.1 ng/mL was a significantly favorable prognostic factor (28). In this study, the significant prognostic factors were the pre-RT PSA level, Gleason score, and seminal vesicle invasion, and were compatible with the former studies. The 5-year BCR-free survival rate was significantly higher than late SRT group, 83.4% and 52.2%, respectively, P=0.007. Surgical margin, PSA doubling time, and post-RP PSA nadir revealed a slight trend, but were not significant predictive factors in univariate or multivariate analysis. This could be due to the fact that this retrospective study consisted of a limited cohort of heterogeneous patients.
There were no published phase III randomized trials directly comparing the outcomes of adjuvant RT and SRT. One meta-analysis evaluated the outcomes between adjuvant RT and SRT to patients with BCR after RP (23). There were a total 2,380 patients in the analysis including 1,192 patients in adjuvant RT arm and 1,188 patients in SRT arm. Adjuvant RT shows significantly favorable results in BCR-free survival compared to SRT (HR: 0.61). Several randomized controlled trials are now ongoing to clarify whether adjuvant RT is superior to SRT (RAVES; EORTC 22043-30041; GETUG-17; RADICALS-RT).
Retrospective data suggested an improvement in BCR-free survival if short-term ADT is added to SRT (29). There were two phase III randomized trials investigating whether the addition of ADT to SRT in patients with PSA failure after RP would improve BCR-free survival and overall survival. In the RTOG 9601 study (30), 760 patients status post RP with pT2-3N0 who had or developed elevated PSA levels from 0.2 to 4.0 ng/mL underwent SRT and were randomly assigned to anti-androgen therapy (24 months of bicalutamide, 150 mg daily) or a placebo, during and after RT. After a median follow-up of 13 years, both significantly improved 12-year overall survival and BCR in the combining ADT with RT group compared with the RT-only group (76.3% vs. 71.3% and 44% vs. 67.9%, respectively). In the GETUG-AFU 16 trial (31), 743 patients who had rising PSA of 0.2 to 2.0 µg/L following RP were randomly assigned to RT alone and RT plus goserelin. Five-year progression-free survival in the RT plus goserelin group was significantly better than in the RT-only group (80% vs. 62%; P<0.0001). The ongoing RADICAL-HD trial compares RT alone, RT plus short course ADT (6 months), and RT plus long course ADT (2 years). In our study, the combined SRT with ADT did not improve the time to biochemical progression compared with SRT-only (P=0.928). The result may be due to the limited number of patients. Only 8 patients received combined modality therapy, though we included patients treated with LHRH agonists and/or anti-androgens. Based on the two randomized control trials, updated practical guidelines (e.g., NCCN guideline) discussed the addition of ADT to SRT (10). However, we have not yet reached consensus on this within our institution.
Five patients (10.4%) received pelvic RT and none of them developed BCR. Three patients (6.3%) developed pelvic LN relapse and they all received prostate fossa irradiation without pelvic RT. However, pelvic RT was not a significant prognostic factor in univariate analysis (HR: 0.04, P=0.347). Again, the result may be due to the limited number of patients. Further studies are needed to verify the ideal field for SRT.
There were only a few studies reported from Asia and from Taiwan. In these studies, the BCR-free survival rate at 5 years was 50–70%. One study from Taiwan reported outcomes of SRT after RP, and the 5-year disease-specific survival and BCR rate were 95% and 60%, respectively (28). The 5-year PSA relapse-free rate was 68.4% in our study, and it is in line with other studies. We demonstrated the outcomes of various prognostic factors including age, performance, initial PSA levels, Gleason scores of surgical specimen, pathologic stages, surgical margin statuses, seminal vesicle invasion, post-RP PSA nadir, pre-RT PSA levels, RT dose, RT field, and whether previously treated with or concurrent with ADT.
This study was limited by its retrospective design, patients from single center, and a relatively small number of patients. Selection bias is another limitation. At our institution, the selection of the post-RP management was mainly dependent on the patient and the urologist. Some patients with adverse risk after RP received adjuvant RT rather than observation first, and some received hormone therapy. These contributed to a selection bias.
Conclusions
SRT was an effective treatment for BCR following RP with tolerable toxicities in Taiwanese patients. The pre-RT PSA level was a prognostic factor for PSA relapse after SRT. Early SRT for patients with pre-RT PSA levels <0.5 ng/mL, Gleason score <8, and non- seminal vesicle invasion were associated with better biochemical-recurrence-free survival. Further randomized controlled trials are required to confirm the efficacy of early SRT following RP for prostate cancer. The use of concurrent ADT with SRT needs further discussion.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: JAL serves as an unpaid editorial board member of Therapeutic Radiology and Oncology from Apr 2020 to Mar 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of China Medical University Hospital (CMUH106-REC1-060). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Health promotion administration ministry of health and welfare Taiwan. Cancer Registry Annual Report, 2013.
- Isbarn H, Wanner M, Salomon G, et al. Long-term data on the survival of patients with prostate cancer treated with radical prostatectomy in the prostate-specific antigen era. BJU Int 2010;106:37-43. [Crossref] [PubMed]
- Roehl KA, Han M, Ramos CG, et al. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol 2004;172:910-4. [Crossref] [PubMed]
- Boorjian SA, Karnes RJ, Crispen PL, et al. Radiation therapy after radical prostatectomy: impact on metastasis and survival. J Urol 2009;182:2708-14. [Crossref] [PubMed]
- Cotter SE, Chen MH, Moul JW, et al. Salvage radiation in men after prostate-specific antigen failure and the risk of death. Cancer 2011;117:3925-32. [Crossref] [PubMed]
- Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol 2007;25:2035-41. [Crossref] [PubMed]
- Tendulkar RD, Agrawal S, Gao T, et al. Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol 2016;34:3648-54. [Crossref] [PubMed]
- Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 2008;299:2760-9. [Crossref] [PubMed]
- Freedland SJ, Rumble RB, Finelli A, et al. Adjuvant and salvage radiotherapy after prostatectomy: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol 2014;32:3892-8. [Crossref] [PubMed]
- NCCN clinical practice guidelines in oncology. Prostate cancer. Version 2, 2017.
- Thompson IM, Valicenti RK, Albertsen P, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol 2013;190:441-49. [Crossref] [PubMed]
- Buskirk SJ, Pisansky TM, Schild SE, et al. Salvage radiotherapy for isolated prostate specific antigen increase after radical prostatectomy: evaluation of prognostic factors and creation of a prognostic scoring system. J Urol 2006;176:985-90. [Crossref] [PubMed]
- Cheung R, Kamat AM, de Crevoisier R, et al. Outcome of salvage radiotherapy for biochemical failure after radical prostatectomy with or without hormonal therapy. Int J Radiat Oncol Biol Phys 2005;63:134-40. [Crossref] [PubMed]
- Lee AK, D'Amico AV. Utility of prostate-specific antigen kinetics in addition to clinical factors in the selection of patients for salvage local therapy. J Clin Oncol 2005;23:8192-7. [Crossref] [PubMed]
- Patel R, Lepor H, Thiel RP, et al. Prostate-specific antigen velocity accurately predicts response to salvage radiotherapy in men with biochemical relapse after radical prostatectomy. Urology 2005;65:942-46. [Crossref] [PubMed]
- Pazona JF, Han M, Hawkins SA, et al. Salvage radiation therapy for prostate specific antigen progression following radical prostatectomy: 10-year outcome estimates. J Urol 2005;174:1282-6. [Crossref] [PubMed]
- Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA 2004;291:1325-32. [Crossref] [PubMed]
- Ward JF, Zincke H, Bergstralh EJ, et al. Prostate specific antigen doubling time subsequent to radical prostatectomy as a prognosticator of outcome following salvage radiotherapy. J Urol 2004;172:2244-8. [Crossref] [PubMed]
- Kwon O, Kim KB, Lee YI, et al. Salvage radiotherapy after radical prostatectomy: prediction of biochemical outcomes. PLoS One 2014;9:e103574 [Crossref] [PubMed]
- Tsan DL, Fan KH, Chen YC, et al. Pre-radiotherapy PSA level as a predictor for biochemical control in prostate cancer patients receiving radiotherapy after radical prostatectomy. Biomed J 2013;36:71-6. [Crossref] [PubMed]
- Yoshida T, Nakayama M, Suzuki O, et al. Salvage radiotherapy for prostate-specific antigen relapse after radical prostatectomy for prostate cancer: a single-center experience. Jpn J Clin Oncol 2011;41:1031-6. [Crossref] [PubMed]
- American Joint Committee on Cancer. AJCC Cancer Staging Manual. 7th edition. 2010.
- Ku JY, Lee CH, Ha HK. Long-term oncologic outcomes of postoperative adjuvant versus salvage radiotherapy in prostate cancer: Systemic review and meta-analysis of 5-year and 10-year follow-up data. Korean J Urol 2015;56:735-41. [Crossref] [PubMed]
- King CR. The timing of salvage radiotherapy after radical prostatectomy: a systematic review. Int J Radiat Oncol Biol Phys 2012;84:104-11. [Crossref] [PubMed]
- Pfister D, Bolla M, Briganti A, et al. Early salvage radiotherapy following radical prostatectomy. Eur Urol 2014;65:1034-43. [Crossref] [PubMed]
- Ploussard G, Staerman F, Pierrevelcin J, et al. Clinical outcomes after salvage radiotherapy without androgen deprivation therapy in patients with persistently detectable PSA after radical prostatectomy: results from a national multicentre study. World J Urol 2014;32:1331-8. [Crossref] [PubMed]
- Briganti A, Wiegel T, Joniau S, et al. Early salvage radiation therapy does not compromise cancer control in patients with pT3N0 prostate cancer after radical prostatectomy: results of a match-controlled multi-institutional analysis. Eur Urol 2012;62:472-87. [Crossref] [PubMed]
- Wang YJ, Huang CY, Hou WH, et al. Dual-timing PSA as a biomarker for patients with salvage intensity modulated radiation therapy for biochemical failure after radical prostatectomy. Oncotarget 2016;7:44224-35. [PubMed]
- Jang JW, Hwang WT, Guzzo TJ, et al. Upfront androgen deprivation therapy with salvage radiation may improve biochemical outcomes in prostate cancer patients with post-prostatectomy rising PSA. Int J Radiat Oncol Biol Phys 2012;83:1493-9. [Crossref] [PubMed]
- Shipley WU, Seiferheld W, Lukka H, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med 2017;376:417-28. [Crossref] [PubMed]
- Carrie C, Hasbini A, de Laroche G, et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol 2016;17:747-56. [Crossref] [PubMed]
Cite this article as: Wu WC, Lai YL, Liang JA. Salvage radiotherapy for biochemical recurrence after radical prostatectomy: experience of a single center. Ther Radiol Oncol 2018;2:3.