
Complicated respiratory motion analysis and new CT-based instruction model to enhance deep inspiration breath hold technique
Introduction
Breast cancer is one of the most common cancers in women in the world. The American Cancer Society estimated 246,660 new diagnosed cases in United States for 2016. In Taiwan, breast cancer is the most common cancer in women and also the fourth cause of cancer death. Study showed that peak age of diagnosed breast cancer of Asian was between 40–50 instead of 60–70 years old in Western countries (1). Health Promotion Administration Ministry of Health and Welfare of Taiwan recently reports the median of breast cancer diagnosed age was 54 years old in 2013, and the number of new diagnosed breast cancer patient gradually increases yearly.
For early stage breast cancer, it is recommended to conduct breast conservative surgery with breast irradiation. Adjuvant radiotherapy has been proven to reduce local failure rate and mortality rate (2,3). Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis proved that radiotherapy avoided one breast cancer death over 15 years for every four avoided local recurrences in 10 years (2). However, EBCTCG meta-analysis also found that there was excess mortality rate for post-BCS group after 15 years follow up. Main causes of the excess mortality for these patients were heart disease and lung disease (2). Kim et al. also showed that women received radiotherapy for breast cancer treatment had a 1.76-fold higher risk of cardiac disease death and 1.33-fold higher risk of lung disease death than those who did not receive radiotherapy (4). Darby et al. revealed that with the increase of heart mean dose per gray, the rate of major coronary event increases 7.4% linearly (5).
For breast cancer patient receiving adjuvant radiotherapy, normal organ radiation exposure should be as low as possible. Several methods including prone position, intraoperative radiotherapy, deep inspiration breath hold technique (DIBH), etc. were developed aiming to decrease lung and heart dose. The purpose of DIBH technique is to increase the lung volume and also the distance between irradiated target and heart, thus the radiation exposure of heart, left anterior descending artery (LAD), and lung will be reduced during treatment (6,7). The DIBH technique can be performed with instruction only or various assistant tools, such as Real-time Position ManagementTM (RPM) system or Active Breathing Coordinator (ABC) devices. In our institution, RPM system is employed yet we noticed that although DIBH reduces the radiation exposure to organ at risk (OAR), the effectiveness varies for each patient. It is noticed that in the treatment some patients have struggled to fulfil the instruction of DIBH technique even with the RPM system. The data in this study was collected from one institution, associated physical factors changing during DIBH was evaluated in order to enhance DIBH effectiveness.
Methods
Left breast cancer patients who received left partial mastectomy and adjuvant radiotherapy with DIBH technique between Sep 1, 2013 and Sep 1, 2014 were included. Patients who received previous chest irradiation and had multiple cancers history were excluded.
Immobilization and CT simulation
Patients were immobilized with customized vacuum bag. Breath hold while deep inspiration was requested. The RPM system was used to monitor patient’s breath hold condition. The marker box with reflective dots was put on the end of chest wall at the level of diaphragm and was tracked by infrared tracking camera. Helical computed tomography (CT) simulation with 2.5mm between each slice by GE-RT590 was performed. Both free breath (FB) CT scan and DIBH CT scan were acquired. CT simulation images were imported into Varian Eclipse version 11 to proceed radiotherapy treatment planning.
Delineation and treatment planning
For each patient, left breast tissue was contoured as clinical target volume (CTV) following Radiation Therapy Oncology Group contouring guideline. The breast tissue volume was contoured from 2nd rib insertion as cranial margin to loss of CT apparent breast tissue as caudal margin. Anterior margin of whole breast tissue was skin. Pectoralis muscle, chest wall muscle, and ribs were excluded as posterior margin. Lateral margin was mid axillary line typically excluding latissimus dorsi muscle, and medial margin was sternal rib junction. Clinical reference on CT was also taken into account while contouring. None of the lymph node region was included in the CTV. The added margin for creating planning target volume (PTV) from CTV was 5 mm. OAR, including ipsilateral left lung, contralateral right lung, heart, and LAD, and target volume were delineated by same radiation oncology resident. The attending physicians responsible for each patient reviewed the contour to confirm the final treatment target volume.
Dose of 50 Gy was prescribed to the PTV. For each patient, two treatment plans using unopposed tangential IMRT technique were generated based on FB CT and DIBH CT images. All plans met the same criteria of treating 95% of PTV to at least 47.5 Gy and maximum hot spots less than 110%. In order to evaluate the effect to dose distribution contributed by DIBH clearly, primary tumor bed boost was not discussed in this study to diminish the uncertain effect caused by different tumor bed location of each patient. Dose of ipsilateral left lung, contralateral right lung, total lung, LAD, and heart was calculated and dose-volume histograms were constructed. Maximum dose (Dmax), minimum dose (Dmin), mean dose (Dmean), the minimum dose received by 30% of the target volume (D30), percentage of organ volume receiving 5 Gy (V5), percentage of organ volume receiving 20 Gy (V20), and percentage of organ volume receiving 30 Gy (V30) were calculated for each OAR.
Measurement of physical factors
CT simulation image was reviewed from cranial to caudal direction. The first appearance of lung tissue on axial view was defined as upper chest wall border. Half way (middle level) between the first appearance of diaphragm and the last image of lung tissue on axial CT was designated as the diaphragm level. The level where either left or right atrium observed first was defined as upper border of heart level. Chest wall was divided into upper and lower part according to upper border of heart.
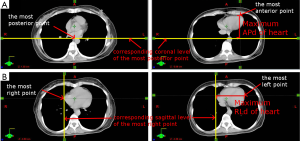
Anterior-to-posterior distances (APd) and right-to-left distances (RLd) of chest wall on both FB and DIBH CT image were measured at 4 levels: (I) half upper chest wall level which was defined as half way between upper chest wall border and upper border of heart; (II) upper border of heart level; (III) half lower chest wall level which was defined as half way between upper border of heart level and diaphragm level; and (IV) diaphragm level, as shown in Figure 1. The lung height of FB and DIBH CT were measured and the extended lung height in DIBH CT image was recorded. As for heart, maximum APd, maximum RLd and total height were measured. First, the most posterior point of heart was found on the axial image which was marked by crosshair, and the most anterior point of heart then was found by scrolling down the image. The distance between the most anterior point of heart to pervious marked coronal level position was calculated and defined as maximum APd shown as Figure 2A. Maximum RLd was defined using the same technique as shown in Figure 2B. Total height of heart was defined as the distance between upper heart border level and the last heart-observable level. Also, the height of heart below the lowest border of treatment target was recorded as free-contour height of heart. The difference of these physical parameters between FB and DIBH CT image were calculated and analyzed.

Analysis
Data were summarized as means and standard deviation. Wilcoxon signed rank test was used to examined the significance of difference. Pearson correlation test was used to examine the correlation significance among each physical factor and OAR radiation exposure volume and dose. This study was approved by the institutional review board as CGH-P104032.
Results
Totally 10 patients were included in this study. The median age of patients was 44 years old. The major cancer stage was stage I and type luminal A was the major molecular subtype. The characteristics of patients were shown as Table 1. All patients received left breast conserving surgery and most of them received sentinel lymph node dissection followed by adjuvant hormone therapy. Treatment detail was listed as Table 2. DIBH significantly reduced Dmax (P=0.047), Dmean (P=0.005), D30 (P=0.037), V5 (P=0.047), V20 (P=0.007), and V30 (P=0.009) of ipsilateral left lung. For contralateral right lung, the only parameter been affected effectively was Dmax (P=0.047). For heart, significant dose reduction of Dmax (P=0.005), Dmean (P=0.005), D25 (P=0.005), V5 (P=0.005), V20 (P=0.008), and V30 (P=0.018) were revealed. DIBH reduced Dmin (P=0.005), Dmax (P=0.005), and Dmean (P=0.005) of LAD significantly shown as Table 3. The dose distribution of FB and DIBH at different heart levels was shown as Figure 3.
Table 1
| Characteristics | No. of patient/value |
|---|---|
| Median age at treatment (years) | 44 [28–59] |
| Breast cancer stage, n [%] | |
| 0 | 1 [10] |
| I | 7 [70] |
| II | 2 [20] |
| Tumor stage, n [%] | |
| 0 | 1 [10] |
| T1 | 8 [80] |
| T2 | 1 [10] |
| Nodal stage, n [%] | |
| N0 | 8 [80] |
| N1 | 2 [20] |
| Histologic type, n [%] | |
| Ductal carcinoma in situ | 1 [10] |
| Invasive ductal carcinoma | 8 [80] |
| Mixed invasive ductal and lobular carcinoma | 1 [10] |
| Molecular subtype, n [%] | |
| Luminal A | 5 [50] |
| Luminal B | 3 [30] |
| Triple negative | 1 [10] |
| HER2 type | 0 [0] |
| Uncertain | 1 [10] |
Table 2
| Treatment | No. of patient [%] |
|---|---|
| LN dissection | |
| Sentinel lymph node dissection | 8 [80] |
| Conventional axillary lymph node dissection | 2 [20] |
| Systemic therapy | |
| Neoadjuvant chemotherapy | 0 [0] |
| Adjuvant chemotherapy | 6 [60] |
| Adjuvant trastuzumab | 2 [20] |
| Adjuvant endocrine therapy | 8 [80] |
| None | 1 [10] |
Table 3
| Organ | Dose/volume of interest | DIBH | FB | P value |
|---|---|---|---|---|
| Ipsilateral lung | Dmax (cGy) | 4,992.8±115.7 | 5,058.0±133.5 | 0.047 |
| Dmean (cGy) | 633.0±139.7 | 746.3±167.7 | 0.005 | |
| D30 (cGy) | 375.0±97.4 | 467.1±168.6 | 0.037 | |
| V5 (%) | 24.5±4.7 | 28.0±6.1 | 0.047 | |
| V20 (%) | 10.9±3.4 | 13.6±4.4 | 0.007 | |
| V30 (%) | 7.4±2.5 | 9.3±3.1 | 0.009 | |
| Contralateral lung | Dmax (cGy) | 401.4±478.0 | 210.3±165.0 | 0.047 |
| Heart | Dmax (cGy) | 2,203.8±1,772.8 | 4,166.7±1,478.6 | 0.005 |
| Dmin (cGy) | 8.3±7.0 | 9.8±6.9 | 0.508 | |
| Dmean (cGy) | 132.7±69.8 | 264.0±165.4 | 0.005 | |
| D25 (cGy) | 136.4±47.2 | 209.8±89.5 | 0.005 | |
| V5 (%) | 2.7±3.2 | 8.0±6.5 | 0.005 | |
| V20 (%) | 0.6±0.9 | 3.3±3.4 | 0.008 | |
| V30 (%) | 0.3±0.6 | 1.9±2.6 | 0.018 | |
| LAD | Dmax (cGy) | 1,882.6±1,726.7 | 3,816.6±1,760.7 | 0.005 |
| Dmin (cGy) | 163.8±77.6 | 479.3±527.3 | 0.005 | |
| Dmean (cGy) | 903.2±872.6 | 2,248.8±1,528.5 | 0.005 |
Data are shown as mean ± standard deviation. DIBH, deep inspiration breath hold; FB, free breath; LAD, left anterior descending artery; Dmax, maximum dose; Dmin, minimum dose; Dmean, mean dose; D30, the minimum dose received by 30% of the target volume; V5, percentage of organ volume receiving 5 Gy; V20, percentage of organ volume receiving 20 Gy; V30, percentage of organ volume receiving 30 Gy.
Effect to OAR irradiated dose contributed by physical factor change under DIBH was further evaluated. Significant positive correlation between the increase of ipsilateral left lung volume and the reduction of all irradiated lung dose parameters except Dmax were revealed. The change of certain defined physical factors, including the increase of APd at level of half upper chest wall, upper heart border level, half lower chest wall level, and extra lung length caused by DIBH, had positive correlation with the reduction of irradiated lung dose as shown in Table 4. The increase of APd of chest wall at upper heart border level, half lower chest wall level, and RLd at diaphragm level showed significant positive correlation with the reduction of Dmax of heart (Pearson correlation r=0.795, 0.835, 0.655; P=0.006, 0.003, and 0.04, respectively) and Dmax of LAD (r=0.788, 0.795, 0.673; P=0.007, 0.006, and 0.033, respectively). The increase of APd at half lower chest wall level, and RLd at diaphragm level had significant positive correlation (r=0.642, 0.682; P=0.045, and 0.03, respectively) with the reduction of Dmean of LAD as shown in Table 5.The increase of free-contour heart height had negative correlation with V30, Dmax of heart, and Dmax of LAD.
Table 4
| Volume/distance discrepancy | Left lung Dmax Dec (Gy) | Left lung Dmean Dec (Gy) | Left lung D30 Dec (Gy) | Left lung V5 Dec (%) | Left lung V20 Dec (%) | Left lung V30 Dec (%) |
|---|---|---|---|---|---|---|
| Total lung V Inc (mL) | 0.477 | 0.914** | 0.818** | 0.935** | 0.883** | 0.754* |
| Left lung V Inc (mL) | 0.5 | 0.877** | 0.774** | 0.923** | 0.837** | 0.702* |
| HalfUpAPd Inc (cm) | 0.502 | 0.839** | 0.670* | 0.810** | 0.728* | 0.823** |
| UpAPd Inc (cm) | 0.674* | 0.784** | 0.579 | 0.626 | 0.720* | 0.809** |
| HalfLowAPd Inc | 0.782** | 0.680* | 0.56 | 0.527 | 0.705* | 0.566 |
| ExtLungHeight (cm) | 0.248 | 0.797** | 0.760* | 0.893** | 0.779** | 0.625 |
| HeartRLd Inc (cm) | −0.346 | −0.648* | −0.649* | −0.724* | −0.61 | −0.485 |
| HeartAPd Inc (cm) | −0.321 | −0.766** | −0.708* | −0.678* | −0.777** | −0.711* |
| FreeContour Inc (cm) | 0.688* | 0.818** | 0.754* | 0.773** | 0.803** | 0.751* |
**, correlation is significant at the 0.01 level (2-tailed); *, correlation is significant at the 0.05 level (2-tailed). DIBH, deep inspiration breath hold technique; Dec, decrease; Inc, increase; V, volume; HalfUpAPd, anterior-posterior diameter of chest wall at half upper chest wall level; UpAPd, anterior-posterior diameter of chest wall at upper border of heart level; HalfLowAPd, anterior-posterior diameter of chest wall at half lower chest wall level; DiaphRLd, right-left diameter of chest wall at diaphragm level; ExtLungHeight, extended lung height of deep inspiration breath hold condition; HeartAPd, maximum anterior-to-posterior diameter of heart; HeartRLd, maximum right-to-left diameter of heart; FreeContour, height of heart below the lowest border of treatment target.
Table 5
| Distance discrepancy | Heart Dmax Dec (Gy) | Heart Dmin Dec (Gy) | Heart Dmean Dec (Gy) | Heart D25 Dec (Gy) | Heart V5 Dec (%) | Heart V20 Dec (%) | Heart V30 Dec (%) | LAD Dmax Dec (Gy) | LAD Dmin Dec (Gy) | LAD Dmean Dec (Gy) |
|---|---|---|---|---|---|---|---|---|---|---|
| HalfUpAPd Inc (cm) | 0.626 | −0.008 | −0.163 | −0.287 | −0.032 | −0.207 | −0.305 | 0.593 | −0.243 | 0.127 |
| UpAPd Inc(cm) | 0.795** | 0.234 | 0.116 | −0.049 | 0.268 | 0.047 | −0.076 | 0.788** | −0.166 | 0.497 |
| HalfLowAPd Inc | 0.835** | 0.263 | 0.165 | 0.02 | 0.321 | 0.083 | −0.07 | 0.795** | −0.228 | 0.642* |
| DiaphRLd Inc (cm) | 0.655* | 0.419 | 0.356 | 0.19 | 0.479 | 0.281 | 0.159 | 0.673* | 0.069 | 0.682* |
| HeartRLd Inc (cm) | −0.373 | 0.084 | 0.206 | 0.175 | 0.149 | 0.262 | 0.284 | −0.215 | 0.012 | 0.18 |
| HeartAPd Inc (cm) | −0.606 | −0.17 | 0.232 | 0.201 | 0.182 | 0.346 | 0.292 | −0.477 | 0.006 | 0.106 |
| FreeContour Inc (cm) | 0.648* | −0.236 | −0.302 | −0.365 | −0.108 | −0.278 | −0.496 | 0.683* | −0.238 | 0.086 |
**, correlation is significant at the 0.01 level (2-tailed); *, correlation is significant at the 0.05 level (2-tailed). DIBH, deep inspiration breath hold technique; Dec, decrease; Inc, increase; HalfUpAPd, anterior-posterior diameter of chest wall at half upper chest wall level; UpAPd, anterior-posterior diameter of chest wall at upper border of heart level; HalfLowAPd, anterior-posterior diameter of chest wall at half lower chest wall level; DiaphRLd, right-left diameter of chest wall at diaphragm level; ExtLungHeight, extended lung height of deep inspiration breath hold condition; HeartAPd, maximum anterior-to-posterior diameter of heart; HeartRLd, maximum right-to-left diameter of heart; FreeContour, height of heart below the lowest border of treatment target.
Discussion
Studies have shown that breast conservative surgery with adjuvant radiotherapy reduces local failure rate (2,3) and mortality rate (3) for early stage breast cancer. Adjuvant radiotherapy, however, also increases excess lung and heart disease over 15 years for these patients (2,5). Efforts to reduce cardiac toxicities attributed to irradiation include prone position, intraoperative radiotherapy, DIBH, etc. DIBH has been proved to reduce heart and lung radiation exposure during treatment effectively (6,8-10), and possible factors and assistant tools that may affect DIBH effectiveness have been investigated (10,11). For instance, excess radiation exposure to intrathoracic organs can be attributed to chest wall irradiation, especially for post-mastectomy group. The DIBH technique therefore contributes more benefits to this patient group compared to post-breast-conservative-surgery group (9). Greater inspiratory lung volume was reported to enhance the benefit of DIBH (10). The correlation between the effectiveness of DIBH and various anatomic characteristics was investigated by Register et al., where only the cardiac mean dose reduction can be predicted with the reduction of heart volume in field (11).
In this study, various physical factors were defined and their changes during DIBH were evaluated in order to enhance DIBH effectiveness. The Dmean and V20 of lung were reduced significantly with the increase of chest wall APd at half of upper chest wall, upper heart border and half of lower chest wall, and extra lung length of DIBH. Also, the increase of chest wall APd at half of upper chest wall and extra lung length of DIBH could reduce D30 and V5 of lung. Among these factors, however, only the increase of chest wall APd at upper heart border and half of lower chest wall could reduce radiation exposure to heart. This result pointed out lung dose reduction majorly relied on changing of chest wall APd at upper chest wall, especially half of upper chest wall level. The reduction of cardiac dose, on the other hand, majorly relied on changing of chest wall APd at lower chest wall including half of lower chest wall level.
Due to chest wall elevation during inspiration, treatment target position will elevate and lower border of heart will descend caused by lung volume expansion and diaphragm descending. The change of heart lower border and the elevation of target contour lower border lead to increase of free-contour height of heart, which significantly reduced Dmax, V30 of heart and Dmax of LAD. Register et al. reported the reduction of heart volume in field (HVIF) was the only factor shows significant correlation in heart mean dose and other cardiac and LAD measurements among other investigated parameters (11). Our data revealed the similar result but further emphasized the importance of rib cage elevation of DIBH.
Respiration is a complicated motion including gliding of rib cage to up and anterior direction, increase of APd and RLd of chest wall, motion of diaphragm, and shape alteration of heart. Different breath pattern such as chest breathing and diaphragmatic breathing will cause different presentation and combination of above factors (12,13). Under complicated respiration, RPM system detects the marker box on the end of chest wall at the level of diaphragm and assists to ensure the reproducibility of DIBH. However, RPM system was not designed to achieve optimal position of DIBH. The marker box detected by RPM can only represent the changing of APd at diaphragm level and part of the changing of half of lower chest wall level. Increase of APd at these two levels had positive correlation with reduction of both lung and heart dose according to our data. Complete change of chest wall cannot be monitored by RPM during DIBH. For instance, APd at half of upper chest wall and upper heart border are also crucial factors to reduce the radiation exposure of lung, yet their change cannot be detected by RPM system. Multiple level monitoring cooperated with RPM system is suggested according to our result for achieving both optimal position and reproducibility. Further delicate monitoring system needs to be constructed.
Our study has several limitations. First, the patient number of this study was small, further investigation with larger number is needed. Second, the PTV margin added in this study was 5 mm which may be modified in different institution or based on each patient condition. Increased PTV margin could lead to increased OAR dose. The result of this study should be interpreted under the PTV 5 mm precondition. Third, the levels we defined for physical factor measurement were based on CT image. However, for clinical practice, body surface marker would be more practical for daily set up. For this reason, we further reviewed our data attempting to construct the connection between body surface marker position and CT image level. Unfortunately, due to large individual difference of body shape such as breast tissue size, shape, position and individualized forced vital capacity leading to different diaphragm position, the attempting was failed. Fourth, all targets were contoured by the same radiation oncology resident according to RTOG guideline and reconfirmed by attending physician responsible for each patient as 2nd radiation oncologist. Since the responsible attending physician may be different for each patient, a third radiation oncologist for reviewing all the contours may be helpful in consistency inspection. All treatment plans were then performed by the same physicist to ensure the treatment planning technique consistency. Fifth, this study only compared the treatment plans of 50 Gy to left whole breast tissue to evaluate the effect to dose distribution contributed by DIBH more clearly. However, breast cancer treatment contained not only 50 Gy to left whole breast tissue but also boost dosage to primary tumor bed. The primary tumor bed boost also increases additional dose to heart and lung tissue. Further investigation of effect from primary tumor bed location and tumor bed boost treatment with larger number of patients will be needed.
In our hospital, DIBH patients were told to perform deep inspiration as much as they can, and the DIBH was monitored passively by RPM system with one-point evaluation. However, there were patients complained about the instruction of DIBH technique being vague. Under the instruction of deep inspiration as much as they can, it’s uncertain that patient would perform chest or diaphragmatic breathing pattern, or if they know how to achieve similar interfractional chest wall motion. RPM system ensures the reproducibility but not designed for achieving optimal position of chest wall during DIBH. Based on our results and also patient complaint during daily practice, we have built a new CT-based instruction model for DIBH treatment. During CT simulation process, a free breath CT was performed first in order to identify suitable monitor levels including half of upper chest level, upper border of heart level, half of lower chest level, and diaphragmatic level. With these identified CT-based levels, corresponding surface position can be marked according to the distances from the set up center. Body surface markers and marker box were then placed on each level for patient education and training. Patient education is the following crucial step. With the CT-based instruction model, patients were taught to identify the markers on their body surface and practice deep inspiration to keep marked levels elevation under RPM system assistance. After confirming the desired breathing pattern has been understood well, patients can practice the breathing pattern at home during waiting for treatment planning. For daily treatment, patient was asked to perform DIBH and each level elevation was confirmed by RPM system as practice first, then the real treatment would begin with marker box back to diaphragm level. The RPM system or other multiple points monitoring system can be utilized when performing DIBH technique. The clear instruction and monitoring can help patients not only proceed reproducible DIBH every day, but also help them to achieve the inspiration goal easier, and further decrease their anxiousness. Accompanied with patients’ active cooperation, the DIBH technique can be carried out more precisely and minimize the excess radiation exposure to intrathoracic organ.
Conclusions
For left breast cancer patients received left partial mastectomy and adjuvant radiotherapy, DIBH technique is considered to reduce unnecessary dose to lung, heart, and LAD. The RPM system provides an easy way to monitor DIBH and is useful to decrease OAR dose. However, respiration is a complicated motion and the current RPM system cannot represent every physical factor change during respiration. A multiple level monitoring system was suggested, which provides a new direction to evaluate DIBH performance and a new way to enhance the effectiveness of DIBH through comprehensive monitoring. Patient education to explain the idealist chest wall expansion condition with DIBH is important and our CT-based instruction model system can provide a clearer instruction. Further investigation of effect from primary tumor bed location and tumor bed boost treatment with larger number of patients will be needed.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2017.12.03). CJW serves as an unpaid editorial board member of Therapeutic Radiology and Oncology from Oct 2017 to Sep 2019. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of the Cathay General Hospital as CGH-P104032. The need for informed consent was waived by the Institutional Review Board of the Cathay General Hospital (CGH-P104032) and retrospective data was collected after receiving approval from the Institutional Review Board of the Cathay General Hospital (CGH-P104032).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leong SP, Shen ZZ, Liu TJ, et al. Is breast cancer the same disease in Asian and Western countries? World J Surg 2010;34:2308-24. [Crossref] [PubMed]
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. [Crossref] [PubMed]
- Whelan TJ, Julian J, Wright J, et al. Does locoregional radiation therapy improve survival in breast cancer? A meta-analysis. J Clin Oncol 2000;18:1220-9. [Crossref] [PubMed]
- Bouillon K, Haddy N, Delaloge S, et al. Long-term cardiovascular mortality after radiotherapy for breast cancer. J Am Coll Cardiol 2011;57:445-52. [Crossref] [PubMed]
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. [Crossref] [PubMed]
- Hayden AJ, Rains M, Tiver K. Deep inspiration breath hold technique reduces heart dose from radiotherapy for left-sided breast cancer. J Med Imaging Radiat Oncol 2012;56:464-72. [Crossref] [PubMed]
- Joo JH, Kim SS, Ahn SD, et al. Cardiac dose reduction during tangential breast irradiation using deep inspiration breath hold: a dose comparison study based on deformable image registration. Radiat Oncol 2015;10:264. [Crossref] [PubMed]
- Comsa D, Barnett E, Le K, et al. Introduction of moderate deep inspiration breath hold for radiation therapy of left breast: Initial experience of a regional cancer center. Pract Radiat Oncol 2014;4:298-305. [Crossref] [PubMed]
- Lin A, Sharieff W, Juhasz J, et al. The benefit of deep inspiration breath hold: evaluating cardiac radiation exposure in patients after mastectomy and after breast-conserving surgery. Breast Cancer 2017;24:86-91. [Crossref] [PubMed]
- Tanguturi SK, Lyatskaya Y, Chen Y, et al. Prospective assessment of deep inspiration breath-hold using 3-dimensional surface tracking for irradiation of left-sided breast cancer. Pract Radiat Oncol 2015;5:358-65. [Crossref] [PubMed]
- Register S, Takita C, Reis I, et al. Deep inspiration breath-hold technique for left-sided breast cancer: An analysis of predictors for organ-at-risk sparing. Med Dosim 2015;40:89-95. [Crossref] [PubMed]
- Laurin LP, Jobin V, Bellemare F. Sternum length and rib cage dimensions compared with bodily proportions in adults with cystic fibrosis. Can Respir J 2012;19:196-200. [Crossref] [PubMed]
- White BM, Zhao T, Lamb J, et al. Quantification of the thorax-to-abdomen breathing ratio for breathing motion modeling. Med Phys 2013;40:063502 [Crossref] [PubMed]
Cite this article as: Nien HH, Peng CP, Tsai YL, Yu PC, Sung SY, Lui LT, Wu CJ, Shaw S. Complicated respiratory motion analysis and new CT-based instruction model to enhance deep inspiration breath hold technique. Ther Radiol Oncol 2018;2:2.


