
Preliminary study of integrating pretreatment and early follow-up FDG-PET in patients with localized nasal natural killer/T-cell lymphoma receiving concurrent chemoradiotherapy
Introduction
Natural killer/T-cell lymphoma (NKTL) accounts for less than 1% of lymphomas in Europe and North America, but it occurs more frequently with 6% to 15% of lymphomas in East Asia and Latin America (1-4). In Taiwan, NKTL constitutes 10.5% to 12.2% of lymphomas, and there are 350 to 380 new NKTL cases per year (5). Nasal cavity is the most common site of the extranodal NKTL, and 70% to 90% of patients with nasal NKTL present with early-stage localized disease (4,6,7).
Based on the retrospective studies before 2000s, radiotherapy alone with doses >50 Gy via conventional 2-domensional (2D) technique was the mainstay treatment for stage IE/IIE localized nasal NKTL. In the era of 3-dimensional conformal radiotherapy (3DCRT) in 2000s, combined modalities with concurrent or sequential chemoradiotherapy for localized nasal NKTL provided promising results with 80% to 90% complete response (CR) rate and 70% to 80% 3-year overall survival rate in the prospective phase I/II trials in Japan, Korea, and China (3,8-10). Because of the rarity of nasal NKTL and relative small patient numbers of these non-randomized trials, the optimal treatment strategy of the disease is still controversial (2).
Fluorine-18-fluorodeoxyglucose positron emission tomography with computed tomography (FDG-PET/CT) scan serves as a useful tool for cancer staging, radiotherapy target volume delineation, and treatment response assessment. FDG-PET-based radiotherapy planning has been widely applied in the treatment for lung cancers, head and neck cancers. However, it is not well-defined in nasal NKTL (11,12).
Here we aimed to evaluate early responses to concurrent chemoradiotherapy (CCRT) for patients with localized nasal NKTL and integrate pretreatment and posttreatment FDG-PET/CT scans for target delineation and response assessment.
Methods
Patients and evaluations
We retrospectively reviewed patients who were histologically-diagnosed and clinically staged I/II extranodal nasal NKTL in Far Eastern Memorial Hospital from January 2014 to December 2015, with the approval of the Institutional Review Board (IRB approval no: FEMH-IRB-104171-E). Patients receiving CCRT as the primary treatment were included. Patients who had previous cancer history or second primary malignancy were excluded. Pretreatment evaluations including a complete history review, physical examination, complete blood counts, biochemistry tests, liver function tests, blood tumor markers, plasma Epstein-Barr virus (EBV) DNA loads, nasopharyngoscopy with biopsies, bone marrow aspiration, esophagoscopy, abdominal ultrasonography, and contrast-enhanced CT scans were recorded. FDG-PET/CT scans were performed before the treatment and at 1 month following CCRT. The disease was staged according to the Ann Arbor staging system. Part of the study data were based on Cancer Registry database of our hospital.
FDG-PET/CT scans
FDG was injected in the PET Center of Far Eastern Memorial Hospital. All patients fasted for at least 4 hours before FDG injection. The blood glucose levels were less than 140 mg/dL. The injected dose was adjusted according to the body weight (5 MBq/kg, 0.135 mCi/kg). A 30-minute whole-body PET scan from skull to thigh was performed on a PET/CT scanner (Discovery VCT, GE Medical Systems, Milwaukee, WI). The emission data were acquired in 3D mode, and axial field of view. The resolutions of the scanner were 15.7 cm and 4.8 mm full width at half maximum, respectively.
A low-dose whole-body CT was performed for attenuation correction of the emission data and anatomic correlation. No intravenous (IV) or oral contrast agents were given in the series. Both PET and CT scans were performed for patients under normal tidal breathing. From the raw emission data collected, the images were reconstructed by iterative reconstruction with CT-derived attenuation correction using the ordered subsets expectation maximization algorithm.
Quantitative data analyses were derived from SUV, which were normalized to body weight and calculated using data from the attenuation-corrected images by the automatic software. The SUVmax of the lesions were then calculated and analyzed.
Radiotherapy
All patients received external beam radiotherapy with 6 MV photons via image-guided helical tomotherapy (Tomotherapy Inc., Madison, WI) at our department of radiation oncology.
To integrate FDG-PET metabolic information in the radiotherapy planning system, FDG-PET/CT simulation were performed simultaneously in the same PET/CT scanning room, with thermoplastic mask-based immobilization for the patients.
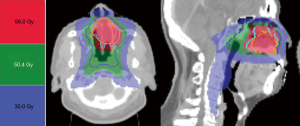
The radiotherapy dose was 56 Gy in 28 fractions to gross disease and 50.4 Gy in 28 fractions to the involved field using intensity-modulated radiation therapy-simultaneous integrated boost (IMRT-SIB) technique (2.0 Gy and 1.8 Gy per day; 5 consecutive days per week). The involved field of nasal NKTL was defined as nasal cavity, nasopharynx, adjacent paranasal sinuses for primary tumors, and involved neck nodal region(s) for stage II disease.
In the process of target volume delineation, radiation oncologists contoured the gross and clinical-biological target volumes according to the integrated fusion images of FDG-PET and simulation CT in the radiotherapy treatment planning system. The gross target volume of 56 Gy (GTV_56) was visible gross tumor contoured on simulation CT slices. The clinical target volume of 56 Gy (CTV_56) consisted of GTV_56 plus FDG-avid volumes. The clinical target volume of 50.4 Gy (CTV_50.4) consisted of the involved field, blurred FDG area and CTV_56 with a 3 to 5 mm expansion. To manage interfraction motions and setup uncertainties, the planning target volumes of 56 Gy and 50.4 Gy (PTV_56 and PTV_50.4) were designed from the CTV_56 plus a 3-mm expansion and CTV_50.4 plus a 5-mm expansion, respectively.
If setup-errors of the patient more than PTV margins were found on the helical megavoltage CT images of tomotherapy, an adaptive re-planning would be required to improve the accuracy of radiation dose distribution to the targeted areas.
All radiotherapy plans in this study were optimized with at least 95% of the PTV covered by the prescribed doses and with the maximum dose less than 110% of the prescribed dose. The dose constraints for organs at risk were as follows: (I) brainstem: a maximum dose of less than 54 Gy; (II) spinal cord: a maximum dose of less than 45 Gy; (III) optic chiasm and optic nerves: a maximum dose of less than 45 Gy; (IV) lens: a maximum dose of less than 10 Gy; (V) bilateral parotid glands: a mean dose of less than 30 Gy, and a median dose less than 26 Gy in each gland; (VI) inner ears: a mean dose less than 45 Gy; (VII) eye orbits: a maximum dose of 45 Gy and a mean dose less than 35 Gy.
Chemotherapy
The concurrent chemotherapy was delivered to patients for 3 cycles during radiotherapy, with repeated every 3 weeks. Chemotherapy regimen consisted of dexamethasone (40 mg/day intravenously on day 1 to 3), etoposide (67 mg/m2 intravenously on day 1 to 3), ifosfamide (1.0 g/m2 intravenously on day 1 to 3), and carboplatin (200 mg/m2 intravenously on day 1).
Follow-up
All patients were evaluated every week during the courses of CCRT. Toxicities were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Patients received regular follow-ups by our multimodality treatment team every 3 months. Post-treatment CT or magnetic resonance imaging (MRI) of head and neck were done at 1, 3, and 6 months following completion of the 3rd cycle of CCRT. Follow-up visits included history review, physical examination, serum biochemistry, and other tests as clinically indicated. For the suspicious lesion on FDG-PET, CT or MRI images, nasopharyngoscopy with biopsies was performed to rule out any residual tumor or recurrence. Responses to CCRT and treatment-related toxicities were analyzed.
Results
Patient characteristics
Four male patients were identified in this retrospective study. Characteristics of patients and results of CCRT were summarized in Table 1. The median age at diagnosis of patients was 61 years old (range, 42–63 years old). Of the four patients, 2 (50%) had stage IE and 2 (50%) had stage IIE disease with multiple neck nodes. The in situ hybridization for EBV-encoded RNA (ISH-EBER) status was detected in four patients. The initial plasma EBV DNA loads were detectable in 2 (50%) patients, and both of their EBV DNA loads became undetectable after treatment.
Table 1
| Characteristics | Patient #1 | Patient #2 | Patient #3 | Patient #4 |
|---|---|---|---|---|
| Age (years) | 63 | 62 | 60 | 42 |
| Gender | Male | Male | Male | Male |
| Tumor involvement | Nasal cavity oropharynx | Nasal cavity oropharynx | Nasal cavity nasopharynx | Nasal cavity nasopharynx |
| Initial neck node(s) | Multiple | Multiple | Absent | Absent |
| B symptoms | Present | Absent | Absent | Absent |
| Elevation of serum LDH | Present | Absent | Absent | Absent |
| Performance status score | 0 | 1 | 0 | 0 |
| Immunohistochemical features | CD3+, CD30+, CD20−, CD56 | CD3−, CD20−, CD56+ | CD3+, CD5+, CD56+ | CD3−, CD20−, CD56+ |
| ISH-EBER status | EBER (+) | EBER (+) | EBER (+) | EBER (+) |
| Pre-CCRT plasma EBV DNA load | Detectable | Detectable | Undetectable | Undetectable |
| Post-CCRT plasma EBV DNA load | Undetectable | Undetectable | Undetectable | Undetectable |
LDH, lactic dehydrogenase; NKTL, natural killer/T cell lymphoma; ISH-EBER, in situ hybridization for EBV-encoded small RNA; CCRT, concurrent chemoradiotherapy; EBER, EBV-encoded RNA; EBV, Epstein-Barr virus.
From the reports of the radiation treatment planning system, the mean tumor volume of these patients was 25.4 cm3. The mean doses of GTV_56, CTV_56, and CTV_50.4 were 58.09 Gy, 58.12 Gy, and 55.30 Gy. Radiation doses to organs at risk were within acceptable tolerances. The mean doses surrounding organs were as follows: right parotid glands, 18.63 Gy; left parotid glands, 18.52 Gy; larynx 18.45 Gy; pharynx 31.02 Gy. The detailed quantitative dose indices for tumors and organs at risk of the patients were listed in Table 2. The representative target volume delineation and radiation dose distribution were shown in Figure 1.
Table 2
| Indices | Patient #1 | Patient #2 | Patient #3 | Patient #4 |
|---|---|---|---|---|
| GTV_56 (cm3) | 27.85 | 25.69 | 36.05 | 12.04 |
| Mean dose of GTV_56 (Gy) | 58.18 | 57.18 | 58.80 | 58.19 |
| CTV_56 (cm3) | 61.19 | 48.15 | 82.22 | 30.87 |
| Mean dose of CTV_56 (Gy) | 58.21 | 57.18 | 58.80 | 58.30 |
| CTV_50.4 (cm3) | 234.60 | 337.09 | 290.35 | 274.53 |
| Mean dose of CTV_50.4 (Gy) | 54.98 | 55.03 | 56.24 | 54.95 |
| Brainstem, maximum dose (Gy) | 25.37 | 31.03 | 36.82 | 33.87 |
| Spinal cord, maximum dose (Gy) | 16.86 | 29.22 | 20.27 | 28.35 |
| Optic chiasma, maximum dose (Gy) | 25.97 | 20.15 | 20.69 | 13.67 |
| Right optic nerve, maximum dose (Gy) | 32.23 | 33.13 | 43.29 | 18.46 |
| Left optic nerve, maximum dose (Gy) | 32.85 | 36.73 | 40.05 | 23.81 |
| Right lens, maximum dose (Gy) | 3.14 | 5.77 | 7.44 | 4.03 |
| Left lens, maximum dose (Gy) | 2.90 | 5.03 | 7.05 | 4.35 |
| Right eyeball, mean dose (Gy) | 5.81 | 11.17 | 13.38 | 7.45 |
| Left eyeball, mean dose (Gy) | 5.61 | 12.80 | 10.40 | 7.79 |
| Right parotid gland, mean dose (Gy) | 18.10 | 18.76 | 18.63 | 19.03 |
| Left parotid gland, mean dose (Gy) | 20.08 | 17.80 | 17.25 | 18.94 |
| Larynx, mean dose (Gy) | 16.18 | 24.17 | 14.60 | 18.85 |
| Pharynx, mean dose (Gy) | 32.79 | 33.61 | 25.02 | 32.64 |
GTV_56, gross target volume of 56 Gy; CTV_56, clinical target volume of 56 Gy; CTV_50.4, clinical target volume of 50.4 Gy.
All patients tolerated well to the IMRT-SIB (56 and 50.4 Gy) via image-guided tomotherapy and three cycles of chemotherapy without interruption during the courses of CCRT. The mean duration of radiation therapy was 39.5±2.6 days (range, 38.0–43.0 days).
Treatment responses and toxicities
At 1 month following completion of CCRT (completion of the 3rd cycle of chemotherapy), all 4 (100%) patients achieved CR by CT, MRI images and FDG-PET metabolic criteria. The treatment responses to CCRT by utilizing FDG-PET/CT were listed in Table 3.
Table 3
| Responses | Patient #1 | Patient #2 | Patient #3 | Patient #4 |
|---|---|---|---|---|
| Metabolic response of nasal tumor | ||||
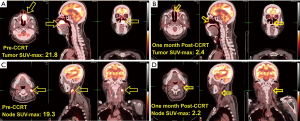
| Pre-CCRT SUVmax | 18.0 | 21.8 | 13.1 | 20.1 |
| Post-CCRT SUVmax | 2.7 | 2.4 | 2.5 | 1.9 |
| Metabolic response of neck node | ||||
| Pre-CCRT SUVmax | 5.0 | 19.3 | N/A | N/A |
| Post-CCRT SUVmax | 2.3 | 2.2 | N/A | N/A |
| Response to CCRT | CR | CR | CR | CR |
CCRT, concurrent chemoradiotherapy; PDG-PET/CT, fluorine-18-fluorodeoxyglucose positron emission tomography with computed tomography; SUVmax, maximum standardized uptake value; N/A, not applicable; CR, complete response.
In the FDG-PET metabolic responses between pre-CCRT and post-CCRT on these patients, the mean of SUVmax of the primary tumors significantly declined from 18.25±3.77 (range, 13.10–21.80) to 2.38±0.34 (range, 1.90–2.70).
Representative images of metabolic CR of nasal tumors and nodes on FDG-PET/CT scans were shown in Figure 2A-D.
The common toxicities of these four patients were leukopenia (grade 2, n=1; grade 3, n=2) and oral mucositis (grade 2, n=3; grade 3, n=1). There were no grade 4 toxicities or treatment-related deaths during CCRT. Treatment-related toxicities of CCRT were acceptable and listed in Table 4.
Table 4
| Toxicities | Patient #1 (grade) | Patient #2 (grade) | Patient #3 (grade) | Patient #4 (grade) |
|---|---|---|---|---|
| Leukopenia | 3 | 3 | 2 | 0 |
| Anemia | 1 | 0 | 0 | 0 |
| Thrombocytopenia | 2 | 0 | 0 | 0 |
| Mucositis | 2 | 2 | 3 | 2 |
| Dermatitis | 0 | 1 | 0 | 2 |
| Weight loss | 0 | 1 | 1 | 2 |
| Nausea | 0 | 0 | 1 | 1 |
Toxicities were graded according to the Common Terminology Criteria for Adverse Events version 3.0. CCRT, concurrent chemoradiotherapy.
At a median follow-up of 29 months (range, 21–33 months), four (100%) patients are currently alive in durable complete remission and disease-free status.
Discussion
The optimal treatment modality for stage IE/IIE nasal NKTL such as radiotherapy alone, concurrent, or sequential chemoradiotherapy and radiation doses were not well-defined (2). In previous studies employing radiotherapy alone for localized nasal NKTL patients, the radiation dose of 50 Gy or more (range, 50–70 Gy) might be required to improve in-field control and response rates. The initial CR rate and 5-year overall survival rate of radiotherapy alone were 60% and 30%, respectively. However, the incidences of local-regional and systemic failure were as high as 25% to 40% in the strategy of radiotherapy alone (2,7).
Based on phase I and II trials investigating combined modalities, including CCRT in Japan or sequential chemoradiotherapy in China and Korea, the radiation doses ranged from 40 to 60 Gy were delivered by 2D or 3DCRT techniques. Combined modalities achieved satisfactory CR rates and survivals with acceptable toxicities (3,8-10).
Radiotherapy techniques have greatly advanced since previous studies of treatment for nasal NKTL after 2000s (1,7-10,13,14). IMRT or volumetric modulated arc therapy (VMAT) make it possible to deliver a higher radiation dose to the tumor while sparing unnecessary doses to the surrounding normal tissues (15-17). Helical tomotherapy (Tomotherapy Inc., Madison, WI) is an image-guided IMRT equipment which can further deliver highly-conformal radiation doses to the tumor precisely and minimize doses to critical organs for head and neck malignancies (18,19).
Our novel approach utilized image-guided radiotherapy with integration of FDG-PET-guidance in the radiation treatment planning and initial response assessment for patients with localized nasal NKTL. In our study, we utilized a modern IMRT-SIB technique in the setting of CCRT, with 56 Gy to gross disease and 50.4 Gy to the involved filed simultaneously. Furthermore, IMRT-SIB technique via image-guided tomotherapy provided adequate radiation dose coverage to tumors and minimized dose to normal organs. Our preliminary results of a 100% CR rate were comparable to the studies employing combined modalities. Meanwhile, the most common grade 3 toxicities such as leukopenia (50%) and mucositis (25%) in our study were also acceptable as compared to previous reports of combined chemoradiotherapy (1,3,8,10).
PET-guided delineation and dose escalation of target volumes are still not well-defined in nasal NKTL (11,12). In a previous meta-analysis of eight studies, the pooled sensitivity and specificity in the diagnosis of 135 NKTL patients were 0.95 (95% CI: 0.89–0.98) and 0.40 (95% CI: 0.09–0.78), respectively (20). Another retrospective study focused on prognostic value of the interim (n=62) and post-therapy (n=47) FDG-PET/CT in patients with NKTL, the concordance between clinical CR on CT or MRI and interim PET negativity was 89.5%; the positive predictive value (PPV), negative predictive value (NPV), and accuracy of interim PET in predicting progression-free survival (PFS) were 77.8%, 76.5%, and 77.4%. Of the post-therapy FDG-PET/CT, the concordance between clinical CR and post-therapy PET negativity was 93.5%; the PPV, NPV, and accuracy in predicting PFS were 88.9%, 58.6% and 70.2% (21). In our study, the pretreatment and early follow-up FDG-PET/CT scans served as a useful tool for NKTL staging, radiotherapy target volume delineation, and treatment response assessment. Furthermore, the integration of FDG-PET and simulation CT images in our radiotherapy treatment planning offered biological and metabolic information for delineation of FDG-avid tumor volumes. Based on the PET-guided radiotherapy treatment planning, radiation oncologists could precisely delineate the target volume of tumors and nodes. Also, the concordance of post-treatment FDG-PET, MRI and CT scans provided helpful information for assessing treatment responses. With modern radiotherapy techniques including FDG-PET-guided delineation, IMRT-SIB planning and image-guided helical tomotherapy, we could accurately deliver conformal radiation doses to the target while sparing surrounding normal organs including brain, spinal cord, nerve, parotid glands, oral mucosa and skin (15-19,22).
Although our results revealed durable initial responses and acceptable toxicities, there were some limitations including relative small numbers of patients and retrospective non-randomized nature because of the rarity of the disease. Further clinical trials and long-term survival follow-ups are required to confirm the validity and reliability of these modern tools.
Conclusions
Our results suggest that CCRT with modern image-guided helical tomotherapy provides a durable initial CR and tolerable side effects for patients with localized nasal NKTL. Integrated FDG-PET-guided radiotherapy is feasible for target delineation and response assessment. Our novel approach is worthy of further investigation, and long-term survival follow-ups are warranted.
Acknowledgments
Funding: This work was partially supported by grants from NYMU-FEMH Joint Research Program (NYMU-FEMH 105FN04 and NYMU-FEMH 106DN02).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This work was approved by the Institutional Review Board of Far Eastern Memorial Hospital (IRB approval No. FEMH-IRB-104171-E). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jiang M, Zhang H, Jiang Y, et al. Phase 2 trial of "sandwich" L-asparaginase, vincristine, and prednisone chemotherapy with radiotherapy in newly diagnosed, stage IE to IIE, nasal type, extranodal natural killer/T-cell lymphoma. Cancer 2012;118:3294-301. [Crossref] [PubMed]
- Tse E, Kwong YL. How I treat NK/T-cell lymphomas. Blood 2013;121:4997-5005. [Crossref] [PubMed]
- Wang L, Wang ZH, Chen XQ, et al. First-line combination of gemcitabine, oxaliplatin, and L-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extranodal natural killer/T-cell lymphoma. Cancer 2013;119:348-55. [Crossref] [PubMed]
- Yang Y, Zhu Y, Cao JZ, et al. Risk-adapted therapy for early-stage extranodal nasal-type NK/T-cell lymphoma: analysis from a multicenter study. Blood 2015;126:1424-32. [Crossref] [PubMed]
- Taiwan Cancer Registry Annual Report. Health Promotion Administration, Ministry of Health and Welfare, Taiwan; 2009, 2010, 2011, 2012. Available online: https://www.hpa.gov.tw/Pages/List.aspx?nodeid=269
- Chim CS, Ma SY, Au WY, et al. Primary nasal natural killer cell lymphoma: long-term treatment outcome and relationship with the International Prognostic Index. Blood 2004;103:216-21. [Crossref] [PubMed]
- Li S, Feng X, Li T, et al. Extranodal NK/T-cell lymphoma, nasal type: a report of 73 cases at MD Anderson Cancer Center. Am J Surg Pathol 2013;37:14-23. [Crossref] [PubMed]
- Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell Lymphoma: consortium for improving survival of lymphoma study. J Clin Oncol 2009;27:6027-32. [Crossref] [PubMed]
- Yamaguchi M, Tobinai K, Oguchi M, et al. Concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: an updated analysis of the Japan clinical oncology group study JCOG0211. J Clin Oncol 2012;30:4044-6. [Crossref] [PubMed]
- Yamaguchi M, Tobinai K, Oguchi M, et al. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol 2009;27:5594-600. [Crossref] [PubMed]
- Grgic A, Nestle U, Schaefer-Schuler A, et al. FDG-PET-based radiotherapy planning in lung cancer: optimum breathing protocol and patient positioning--an intraindividual comparison. Int J Radiat Oncol Biol Phys 2009;73:103-11. [Crossref] [PubMed]
- Minn H, Suilamo S, Seppälä J. Impact of PET/CT on planning of radiotherapy in head and neck cancer. Q J Nucl Med Mol Imaging 2010;54:521-32. [PubMed]
- Cheung MM, Chan JK, Lau WH, et al. Early stage nasal NK/T-cell lymphoma: clinical outcome, prognostic factors, and the effect of treatment modality. Int J Radiat Oncol Biol Phys 2002;54:182-90. [Crossref] [PubMed]
- Li YX, Yao B, Jin J, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol 2006;24:181-9. [Crossref] [PubMed]
- Chang CH, Mok GS, Shueng PW, et al. Fan-shaped complete block on helical tomotherapy for esophageal cancer: a phantom study. Biomed Res Int 2015;2015:959504
- Chen WC, Hwang TZ, Wang WH, et al. Comparison between conventional and intensity-modulated post-operative radiotherapy for stage III and IV oral cavity cancer in terms of treatment results and toxicity. Oral Oncol 2009;45:505-10. [Crossref] [PubMed]
- Gomez DR, Zhung JE, Gomez J, et al. Intensity-modulated radiotherapy in postoperative treatment of oral cavity cancers. Int J Radiat Oncol Biol Phys 2009;73:1096-103. [Crossref] [PubMed]
- Hsieh CH, Kuo YS, Liao LJ, et al. Image-guided intensity modulated radiotherapy with helical tomotherapy for postoperative treatment of high-risk oral cavity cancer. BMC Cancer 2011;11:37. [Crossref] [PubMed]
- Shueng PW, Wu LJ, Chen SY, et al. Concurrent chemoradiotherapy with helical tomotherapy for oropharyngeal cancer: a preliminary result. Int J Radiat Oncol Biol Phys 2010;77:715-21. [Crossref] [PubMed]
- Zhou X, Lu K, Geng L, et al. Utility of PET/CT in the diagnosis and staging of extranodal natural killer/T-cell lymphoma: a systematic review and meta-analysis. Medicine (Baltimore) 2014;93:e258 [Crossref] [PubMed]
- Li YJ, Li ZM, Xia XY, et al. Prognostic value of interim and posttherapy 18F-FDG PET/CT in patients with mature T-cell and natural killer cell lymphomas. J Nucl Med 2013;54:507-15. [Crossref] [PubMed]
- Studer G, Huguenin PU, Davis JB, et al. IMRT using simultaneously integrated boost (SIB) in head and neck cancer patients. Radiat Oncol 2006;1:7. [Crossref] [PubMed]
Cite this article as: Hsu CX, Wang SY, Chang CH, Wu TH, Lin SC, Hsieh PY, Shueng PW. Preliminary study of integrating pretreatment and early follow-up FDG-PET in patients with localized nasal natural killer/T-cell lymphoma receiving concurrent chemoradiotherapy. Ther Radiol Oncol 2018;2:1.


