
Intensity-modulated radiation therapy for a sinonasal intestinal-type adenocarcinoma patient: a rare case report
Introduction
Intestinal-type adenocarcinoma (ITAC) is a very rare tumor, accounting for possibly 1.4% of all the sinonasal and skull base malignancies (1). The major histological types in sinonasal region are squamous cell carcinoma (46%), malignant lymphoma (14%), adenocarcinoma (13%) and malignant melanoma (9%). As its name implies, it has histopathological analogy with gastrointestinal tract adenocarcinomas (2). They are indistinguishable from those of a primary colonic neoplasm and are histologically composed of columnar, goblet and mucus-producing cells. ITAC originates in the ethmoid sinus and the upper section of the nasal cavity, and is likely to involve anterior cranial base. We present a rare case of sinonasal ITAC with right orbital cavity involvement supported by pathologic proof.
Case presentation
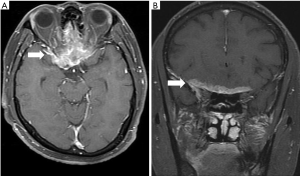
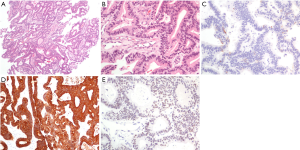
A 62-year-old male businessman was admitted to our hospital for suffering from epistaxis from the past four months and right eye blindness for the past day. He admitted to having worked near a wastewater treatment plant while sometimes inhaling its emitted waste gas. A magnetic resonance imaging (MRI) showed a right sinonasal tumor with intracranial invasion through the cribriform plate, involving bilateral ethmoid sinuses, the left sphenoid sinus, bilateral lower frontal lobes, and the right orbital cavity (Figure 1). A chest radiograph showed no lung lesions. A bone scan showed high probability of local bone invasion from sinus tumor to the head with low probability of distant bone metastasis. Clinical stage was T4bN0M0, stage IVB (AJCC 7th staging). The pathological findings of nasal biopsy revealed intestinal-type moderately differentiated adenocarcinoma, while the immunohistological staining of tumor tissues revealed CK7(−), CK(+), CDX2(+), SMA(−), and p63(−) (Figure 2). He received right fronto-temporal craniotomy with a subfrontal approach for removal of the tumor. The surgical pathology also showed intestinal-type moderately differentiated adenocarcinoma and the resected tumor size was only 1.0 cm × 0.8 cm × 0.6 cm. Post-operative MRI showed residual tumor over the ethmoid sinuses, left sphenoid sinus, bilateral lower frontal lobes, and right orbital cavity.
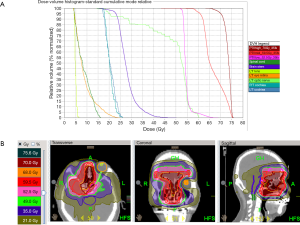
He received radiotherapy 1 month after the operation. The patient adopted a supine posture and underwent 3 mm-sliced computed tomography (CT) scans with delineation of the target volumes. The gross tumor volume (GTV) was specified as the gross extent of tumor as demonstrated by postoperative MRI and CT. Three clinical target volumes (CTV1, CTV2 & CTV3) were defined. The prescribed doses to CTV1, CTV2 and CTV3 were 70, 59.5 and 52.5 Gy in 35 fractions respectively. The CTV1 was defined as the GTV plus 1-cm margin and the CTV2 was defined as an ethmoid sinus, frontal sinuses, nasal cavity, left sphenoid sinus, while the CTV3 was defined as brain invasion surrounding tissue. The planning target volume (PTV) contained a 0.3-cm expansion of CTV (Figure 3). Organs at risk included the chiasm, optic nerve, orbits, lens, brainstem, and parotid glands. The treatment plan was made by the Hi-Art helical tomotherapy, version 2.2.4.1 (Tomotherapy, Inc., Madison, WI) unit.
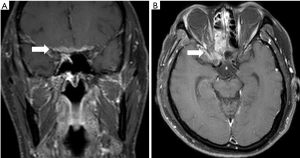
Before radiotherapy treatment, the patient was blind in the right eye and had no light reflex. After radiotherapy, blindness and light reflex improved. The acute complications of radiotherapy were grade I dermatitis and left otorrhea. We examined his tumor status by MRI. After 37 months, MRI still showed partial response of the tumor (Figure 4). After 37 months of follow-up, no progression disease or distant metastasis was found.
Discussion
Surgical resection is the mainstay treatment for sinonasal adenocarcinoma. Radiotherapy can be used preoperatively or postoperatively. Postoperative radiation is usually required due to a high local recurrence rate after surgery alone. However, there are no randomized trials of radiotherapy use for sinonasal adenocarcinoma, not to mention ITAC.
ITAC is a rare tumor. ITAC usually develops from the ethmoid sinus, predominating in males between 40 to 70 years of age (3). ITAC may occur as an occupation-related hazard from exposure to wood dust and leather tanning chemicals (4,5). Interestingly, this patient had no history of wood dust and leather tanning exposure. No report has associated ITAC with waste gas inhalation.
Definitive radiotherapy is less efficient and has more side effects than surgery. It also has poorer outcome than surgery +/− radiotherapy. Jansen et al. (6) reported 73 paranasal sinus carcinoma patients including 12 adenocarcinoma subtypes. Five-year local control rate was 47% with definitive radiation, compared to 65% with radiotherapy + debulking surgery; however, this has no statistical significance. On the other hand, 5-year overall survival was 9% vs. 60% and 5-year disease-free survival was 6% vs. 53%, both with statistical significance. Because of the tumor’s unresectable status, this patient received post-operative RT and he was found to have tumor partial response.
Prophylactic neck irradiation is not necessary. Radiotherapy need only be given to the primary site. A GETTEC
Two studies have reported using intensity-modulated radiotherapy (IMRT) in sinonasal malignancy, but they did not specify histological type. Ghent University Hospital reported 84 patients treated with IMRT to a median dose of 70 Gy in 35 fractions (9). The pathology of these sinonasal tumors was adenocarcinoma in 54 patients, squamous cell carcinoma in 17 patients, esthesioneuroblastoma in 9 patients, and adenoid cystic carcinoma in 4 patients. Among all these patients, 9 patients received definitive IMRT and 75 patients received postoperative IMRT. The 5-year local control and overall survival was 70.7% and 58.5% respectively (9). Daly et al. reported 36 sinonasal cancer patients who were also treated with a median dose of 70 Gy with IMRT (10). Of these cases, there was squamous cell carcinoma in 12 cases, esthesioneuroblastoma in 7 cases, adenocarcinoma in 5 cases, adenoid cystic carcinoma in 5 cases, undifferentiated carcinoma in 5 cases, mucoepidermoid carcinoma in 1 case, and neuroendocrine cancer in 1 case. Four patients received definitive IMRT and 32 patients received postoperative RT. Two patients had persistent disease after radiotherapy. Twelve patients had local progression of disease at a median of 13.4 months (range, 4–31 months). Overall, the 2-year local regional control rate was 62% and 2-year overall survival was 69% (10).
Two studies compared outcomes of sinonasal cancer patients treated with either 3D conformal radiotherapy (3D-CRT) or IMRT. Chen et al. (11) retrospectively reviewed 127 sinonasal patients from 1960 to 2005. Fifty-nine patients were treated by conventional radiotherapy, 45 patients by 3D-CRT; and 23 patients by IMRT. An unexpected observation was that 5-year overall survival rate for sinonasal carcinomas patients had no significant difference: conventional radiotherapy, 3D-CRT, and IMRT was 51%, 57%, and 47% respectively (P=0.60). The author stated that this unexpected result may have been due to cases becoming more complex with time. The incidence of any grade 3 or 4 complications among those treated with conventional radiotherapy, 3D-CRT, and IMRT was 54%, 22%, and 13% respectively. The incidence of any grade 3 or 4 late visual neurologic toxicity among those treated with conventional radiotherapy, 3D-CRT, and IMRT was 20%, 9%, and 0 respectively (P=0.01). A Leuven University Hospital study (12) retrospectively reported 40 sinonasal patients treated by postoperative IMRT vs. 41 sinonasal patients treated by postoperative 3D-CRT. The study reported the incidences of dermatitis (75.0% vs. 97.6%, P=0.003), mucositis (62.5% vs. 97.6%, P<0.001), xerostomia (37.5% vs. 90.2%, P<0.001), headache (45.0% vs. 82.9%, P=0.02), and fatigue (50.0% vs. 78.0%, P=0.008) were significantly lower in the IMRT group (12). IMRT significantly improved disease-free survival (72% vs. 60%, P=0.02) and had a trend for improving 2-year overall survival (89% vs. 73%, P=0.07). No acute or chronic grade 3 or 4 toxicity was reported in the IMRT group (12). According to these two studies, IMRT significantly reduced side effects. This case had acceptable side effects, including grade I dermatitis and temporary otorrhea (12).
No phase III trials have been performed in chemotherapy for ITAC. ITAC could be divided into two groups: wild type TP53 or mutated TP53 tumors by Lieitra et al. (13). After neoadjuvant cisplatin fluorouracil treatment, pathologic complete remission rates were 83% in wild type TP53 tumors and 11% in mutated TP53 tumors respectively (P≤0.0001) (13). However neoadjuvant treatment for locally advanced ITAC is not yet the standard treatment. According to NCCN Guidelines, postoperative systemic therapy/radiotherapy is indicated for patients who have positive margins or intracranial extension in ethmoid sinus cancers (category 2B recommendation).
T classification is an important prognostic factor for sinonasal ITAC; 5-year survival goes down from 80% to 15% when passing from a T1 to a T4b tumor. The study from Ghent University Hospital reported that cribriform plate involvement was the independent factor found on multivariate analyses to predict for local relapse (hazard ratio, 0.058; 95% confidence interval, 0.011–0.297) (9). Those with invasion of the cribriform plate had a median survival of 9.7 months. When cancers involve the cribriform plate, adequate coverage is difficult without exceeding the radiotherapeutic tolerance for the critical structures nearby. This case also had cribriform plate involvement and had acceptable outcome unexpectedly.
In conclusion, we present a rare sinonasal ITAC treated with postoperative radiotherapy. After a 37-month follow-up, no progression disease or distant metastasis was found. Postoperative radiotherapy plays an important role in sinonasal ITAC tumor.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sklar EM, Pizarro JA. Sinonasal intestinal-type adenocarcinoma involvement of the paranasal sinuses. AJNR Am J Neuroradiol 2003;24:1152-5. [PubMed]
- Abecasis J, Viana G, Pissarra C, et al. Adenocarcinomas of the nasal cavity and paranasal sinuses: a clinicopathological and immunohistochemical study of 14 cases. Histopathology 2004;45:254-9. [Crossref] [PubMed]
- Lund VJ, Chisholm EJ, Takes RP, et al. Evidence for treatment strategies in sinonasal adenocarcinoma. Head Neck 2012;34:1168-78. [Crossref] [PubMed]
- Bonzini M, Battaglia P, Parassoni D, et al. Prevalence of occupational hazards in patients with different types of epithelial sinonasal cancers. Rhinology 2013;51:31-6. [PubMed]
- Cantu G, Solero CL, Mariani L, et al. Intestinal type adenocarcinoma of the ethmoid sinus in wood and leather workers: a retrospective study of 153 cases. Head Neck 2011;33:535-42. [Crossref] [PubMed]
- Jansen EP, Keus RB, Hilgers FJ, et al. Does the combination of radiotherapy and debulking surgery favor survival in paranasal sinus carcinoma? Int J Radiat Oncol Biol Phys 2000;48:27-35. [Crossref] [PubMed]
- Choussy O, Ferron C, Védrine PO, et al. Adenocarcinoma of Ethmoid: a GETTEC retrospective multicenter study of 418 cases. Laryngoscope 2008;118:437-43. [Crossref] [PubMed]
- de Gabory L, Maunoury A, Maurice-Tison S, et al. Long-term single-center results of management of ethmoid adenocarcinoma: 95 patients over 28 years. Ann Surg Oncol 2010;17:1127-34. [Crossref] [PubMed]
- Madani I, Bonte K, Vakaet L, et al. Intensity-modulated radiotherapy for sinonasal tumors: Ghent University Hospital update. Int J Radiat Oncol Biol Phys 2009;73:424-32. [Crossref] [PubMed]
- Daly ME, Chen AM, Bucci MK, et al. Intensity-modulated radiation therapy for malignancies of the nasal cavity and paranasal sinuses. Int J Radiat Oncol Biol Phys 2007;67:151-7. [Crossref] [PubMed]
- Chen AM, Daly ME, Bucci MK, et al. Carcinomas of the paranasal sinuses and nasal cavity treated with radiotherapy at a single institution over five decades: are we making improvement? Int J Radiat Oncol Biol Phys 2007;69:141-7. [Crossref] [PubMed]
- Dirix P, Vanstraelen B, Jorissen M, et al. Intensity-modulated radiotherapy for sinonasal cancer: improved outcome compared to conventional radiotherapy. Int J Radiat Oncol Biol Phys 2010;78:998-1004. [Crossref] [PubMed]
- Licitra L, Suardi S, Bossi P, et al. Prediction of TP53 status for primary cisplatin, fluorouracil, and eucovorin chemotherapy in ethmoid sinus intestinal-type adenocarcinoma. J Clin Oncol 2004;22:4901-6. [Crossref] [PubMed]
Cite this article as: Hsu YC, Lu TY, Huang CJ, Huang MY. Intensity-modulated radiation therapy for a sinonasal intestinal-type adenocarcinoma patient: a rare case report. Ther Radiol Oncol 2017;1:3.


